Make It Snow in a Test Tube! Sparkling Crystal Christmas at Home (Ammonium Chloride Recrystallization Experiment)
I’m Ken Kuwako, your science trainer. Every day is an experiment!
The holiday season is finally here! The streets are glowing with beautiful illuminations, and there’s a certain magic in the air. We’ve all paused at least once to admire the intricate frost patterns on a windowpane or the delicate snowflakes dancing down from the sky, thinking, “Wow, that’s beautiful.”
But what if you could recreate that winter magic right on your own desk, inside a tiny test tube? Doesn’t that sound exciting? Today, I’m sharing a stunningly intellectual science experiment that’s perfect for a cozy winter evening. Our star performer is ammonium chloride—a mysterious white powder that creates crystals as delicate as winter fairies.
Grow Your Own Snowflakes: A Festive Christmas Experiment!
Let’s start building your very own “science snow globe!” The preparation is surprisingly simple.
Think of it like brewing a magical potion. Gently add 20g of ammonium chloride to 40mL of water heated to about 70°C. The secret is to stir carefully until everything dissolves and the liquid becomes crystal clear. Pour this “magic water” into a glass container, like a test tube, and then simply wait for it to cool down quietly. Before long, a beautiful winter landscape will begin to unfold inside the glass.
When doing experiments at home, the biggest hurdle is often “dealing with fire.” That’s where a brilliant tip from science education specialist Mr. Eiji Komori comes in: repurpose an old rice cooker! By setting it to “Keep Warm” mode and filling it with water, you transform it into a safe, ideal water bath. This is the electric pot I personally use:
I keep my ammonium chloride pre-measured in test tubes so I can reuse them every year. It’s incredibly convenient.
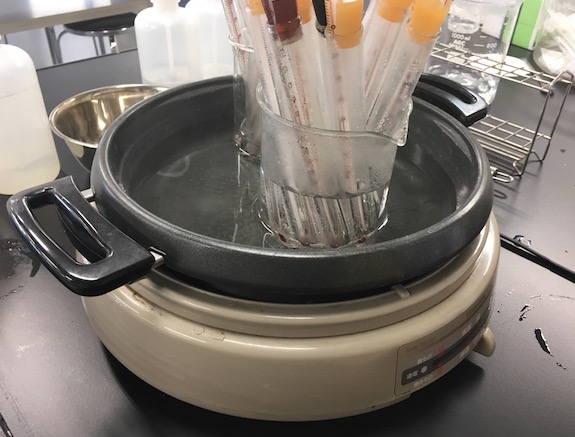
Zojirushi Electric Griddle Pan
By placing the test tubes inside a beaker in the warm water of the electric pot, you get perfect temperature control. Heating over a direct flame carries risks like “bumping” (sudden boiling) or breaking the glass, but this water bath method warms everything gently, making it safe and easy to observe.
So, what kind of scenery awaits? Take a look at this dreamlike moment in the videos below. Watching the crystals grow in the quiet test tube, almost as if they have a will of their own, is so mesmerizing you’ll lose track of time.
Why Does It “Snow”? The Wonder of Recrystallization
Why do these beautiful crystals appear just by cooling a clear liquid? This phenomenon is called “recrystallization.” In fact, it’s a miniature version of how actual snow falls from the sky.
Hot water can dissolve a lot of ammonium chloride. Think of it like a packed commuter train. However, as the solution cools, the capacity of the “train” (the water) decreases. Eventually, it says, “I can’t hold any more! Everyone out!” and the excess ammonium chloride is pushed out.
As these particles lose their place, they begin to hold hands and line up in an orderly fashion. These are the sparkling crystals you see. Natural snow forms in the same way when water vapor in the air cools and gathers into tiny ice particles. You could say that what happens in the test tube is the Earth’s weather reproduced in the palm of your hand.


If you’re thinking, “I want to make even more!” or “I want to see bigger crystals!”, the Narika website is a fantastic resource. By changing the amounts, you can alter how the “snow” falls and grows, allowing you to design your very own unique winter scenery!
A Christmas Tree in the Micro-World
If you peek at these beautiful crystals through a microscope, an even more surprising world—invisible to the naked eye—awaits you.

Can you see the delicate, branching shapes that look like bird feathers or fern leaves? This structure is known as a “dendrite.” The frost patterns that decorate winter windows belong to the same family. It’s as if nature has hidden a tiny “Christmas tree” deep within the microscopic world.
You Can Even Make Gems! The Vast World of Crystals
The world of crystals isn’t limited to ammonium chloride. For instance, using common “alum” (often used in pickling) allows you to grow large, stunning octahedral crystals that look just like gemstones.
This photo shows an impressive alum crystal grown with great patience by students in a science club!

The reason crystal shapes vary so much between substances is that the shapes of the molecules and their “habits” of lining up are unique to each material. You could say the “personality” of the substance is expressed through its shape. Check out the videos below to compare how different substances reveal themselves:
Recrystallization of Potassium Nitrate (Sharp, needle-like crystals rain down!)
Recrystallization of Lead Iodide (Breath-taking golden snow!)
Recrystallization of Alum (Watch as octahedral gems gradually take shape)
What do you think? When you look through the lens of science, winter scenery becomes even more mysterious and beautiful. This Christmas, why not gather around an electric pot in a warm room and let “your own personal miracle” bloom inside a test tube?
Inquiries and Requests
Bringing the wonder and fun of science closer to you! I’ve put together many easy-to-understand guides for fun science experiments you can do at home. Feel free to explore!
My book, based on “Science Notes,” is available now. Click here for details: https://amzn.to/42PMCEL
Learn more about me, Ken Kuwako, here: https://phys-edu.net/wp/?page_id=37
For work requests (writing, lectures, workshops, TV supervision, appearances, etc.): https://phys-edu.net/wp/?page_id=188
Get the latest updates on X: https://x.com/kuwako
![]() Visit the Science Material Channel for experiment videos!
Visit the Science Material Channel for experiment videos!
2月のイチオシ実験!梱包材で遊ぼう!
- 静電気の時期になってきました。子供と一緒に梱包材で盛り上がろう!→ やめられなくなる!静電気実験20
 体中に梱包材をはりつけてみよう!
体中に梱包材をはりつけてみよう!
テレビ番組等・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師等・ショー・その他お知らせ
- 2/20(金)「生徒の進学希望実現支援事業」研究授業@福井県立若狭高等学校 講師
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) サイエンスショー予定
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。

