Where Did the Electricity Go? Uncover the Emotional “V-Shaped Graph” of Neutralization! (Barium Hydroxide × Sulfuric Acid Experiment)
I’m Ken Kuwako, your Science Trainer. Every day is an experiment!
Today, I want to introduce one of the most dramatic and visually satisfying experiments in the chemistry curriculum: the neutralization reaction between barium hydroxide and sulfuric acid. This isn’t just your average chemical reaction. It’s a fascinating process where you can actually watch ions “disappear” from a solution through the movement of an ammeter needle. When you plot the data, it creates a stunningly beautiful V-shaped curve. From preparation tips to the thrilling conclusion, let’s dive into the science behind this “vanishing act”!
Preparation: The Secret is in the “Supernatant”!
Let’s start with the setup. Precise preparation is the first step toward a safe and successful experiment.
1. Preparing the Barium Hydroxide Solution
Once your lab day is set, dissolve plenty of barium hydroxide in water and let it sit undisturbed for a few days. You only want to use the clear supernatant (the liquid at the top). Since barium hydroxide is a strong alkali, please make sure to wear safety goggles. It reacts easily with carbon dioxide in the air to form a white film (barium carbonate), so don’t make it too far in advance. Freshly prepared for each experiment is best. Keep your beakers covered with plastic wrap during storage.

Recommended Amounts (for 4 classes): Adding about 10g of barium hydroxide octahydrate to 220mL of water creates a saturated solution. For a group of 8 students, you only need 5mL x 8 groups = 40mL. For four classes, 160mL is plenty (this calculation includes a bit of a buffer).

On the left is a freshly made batch; on the right is one that sat for about a week. It becomes transparent with a thin film on the surface.
2. Diluting the Sulfuric Acid (The Golden Rule!)
Next, prepare the sulfuric acid. There is one absolute rule here: “Never pour water into concentrated sulfuric acid.” It generates intense heat and can cause the liquid to splatter violently. Always follow the order of “adding concentrated sulfuric acid to water” in small amounts.
Dilution Guide (for 4 classes): Carefully add 3mL of concentrated sulfuric acid to 51mL of water, then dilute this 10-fold to make a total of 540mL (approx. 0.1 mol/L).
Put about 15mL in a test tube and set it out with a pipette to make it easy for students to measure. Also, prepare beakers containing 5mL of the barium hydroxide solution for each group.

Once the prep is done, it’s finally time to start the experiment!
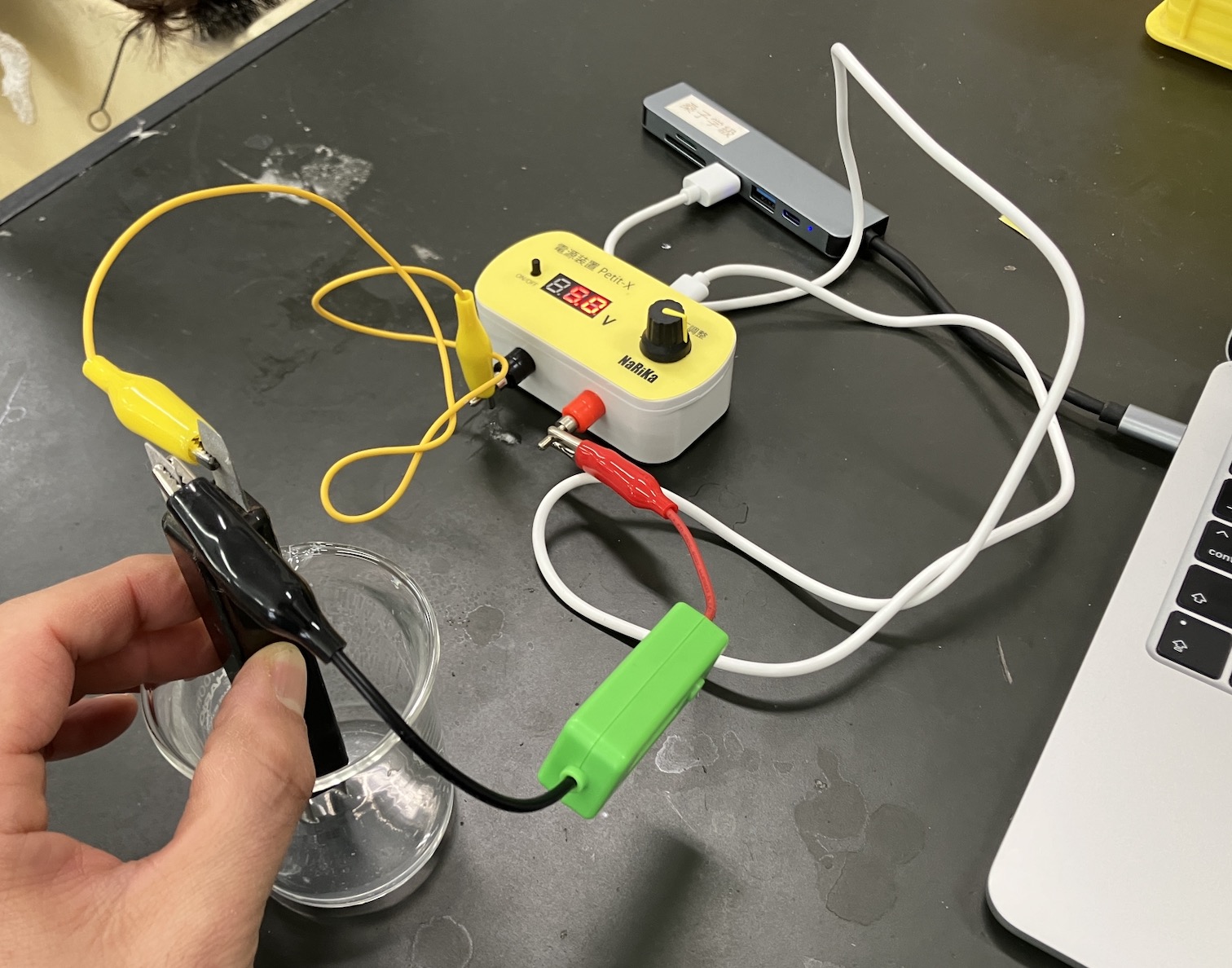
The circuit looks like this. I used the Petit-X mini power supply, which worked great, but any standard power supply will do.
Power On! Setting the Voltage
Connect the power supply and set the voltage to 5V. At this stage, the solution is full of ions, so the current flows strongly.

I used a Petit Meter as the ammeter. Very handy!
The Drop-by-Drop Countdown
Now for the main event. Add the sulfuric acid 1mL at a time, recording the ammeter reading after each drop.
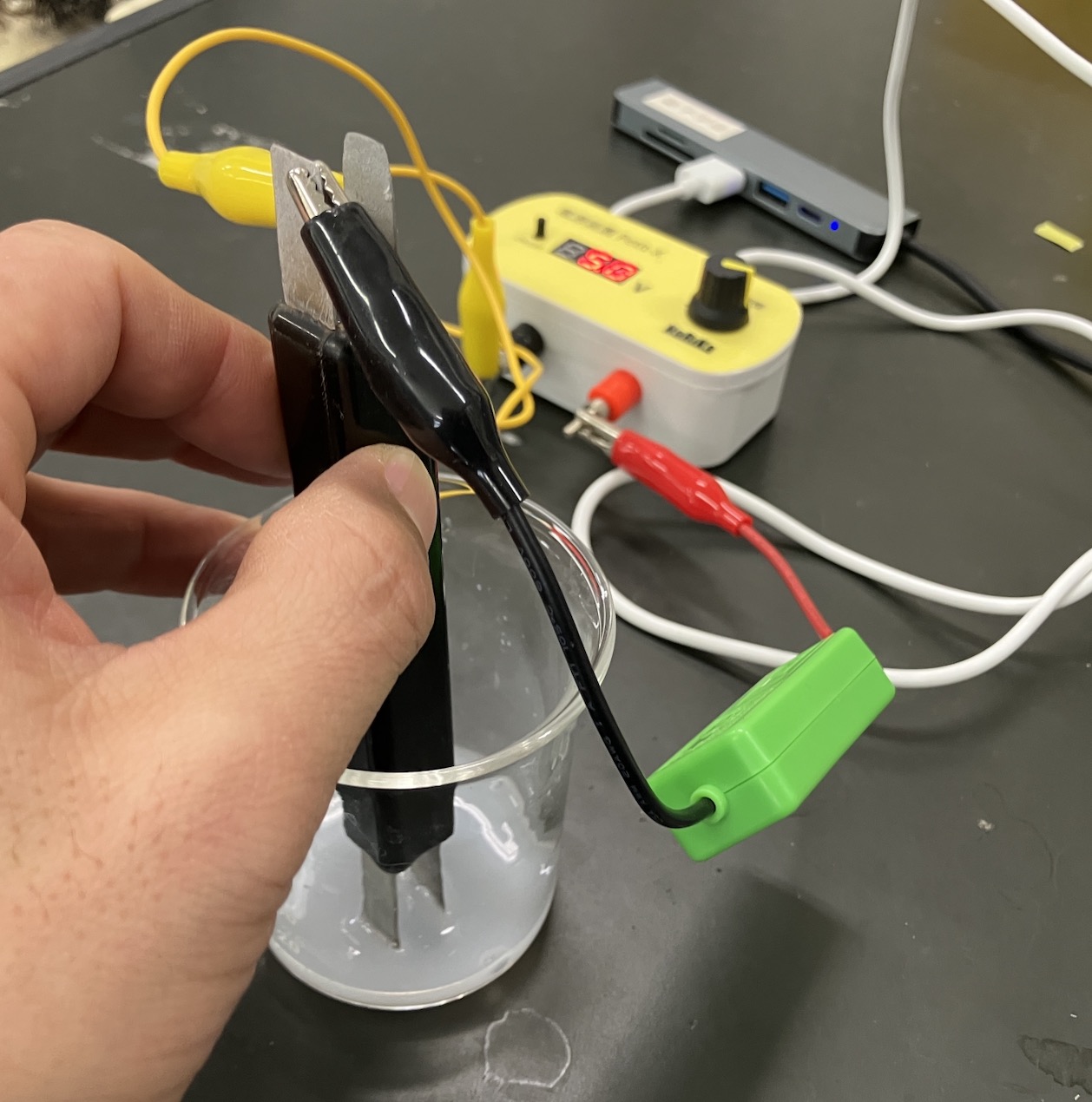
The Magic of the V-Graph: Why Did the Electricity Vanish?
When you plot the data on a graph, a surprising result awaits.
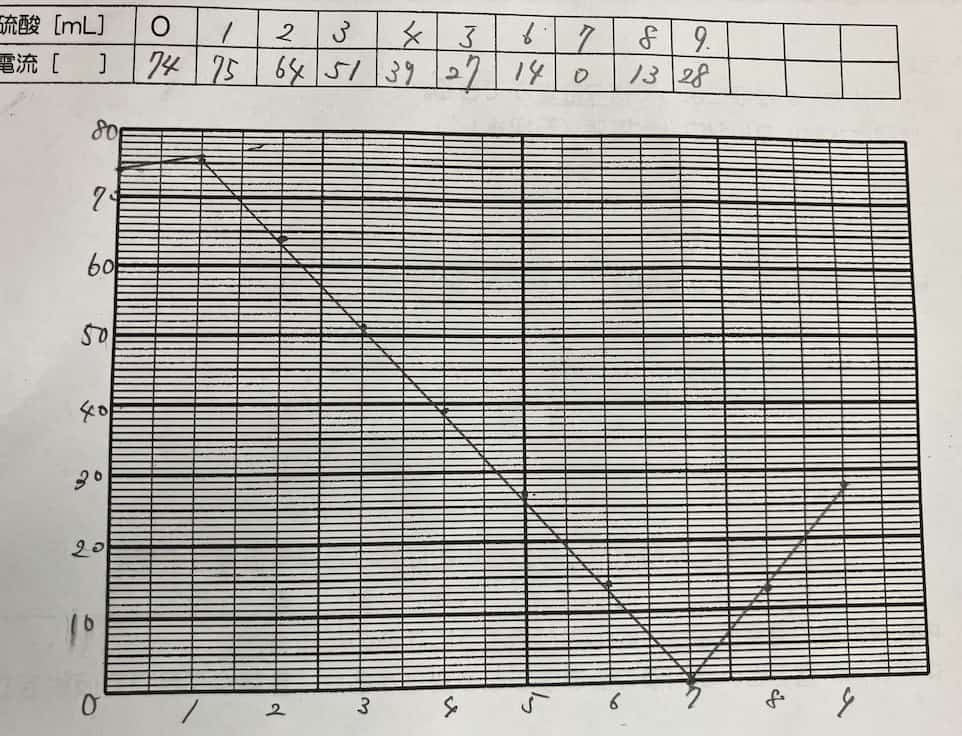
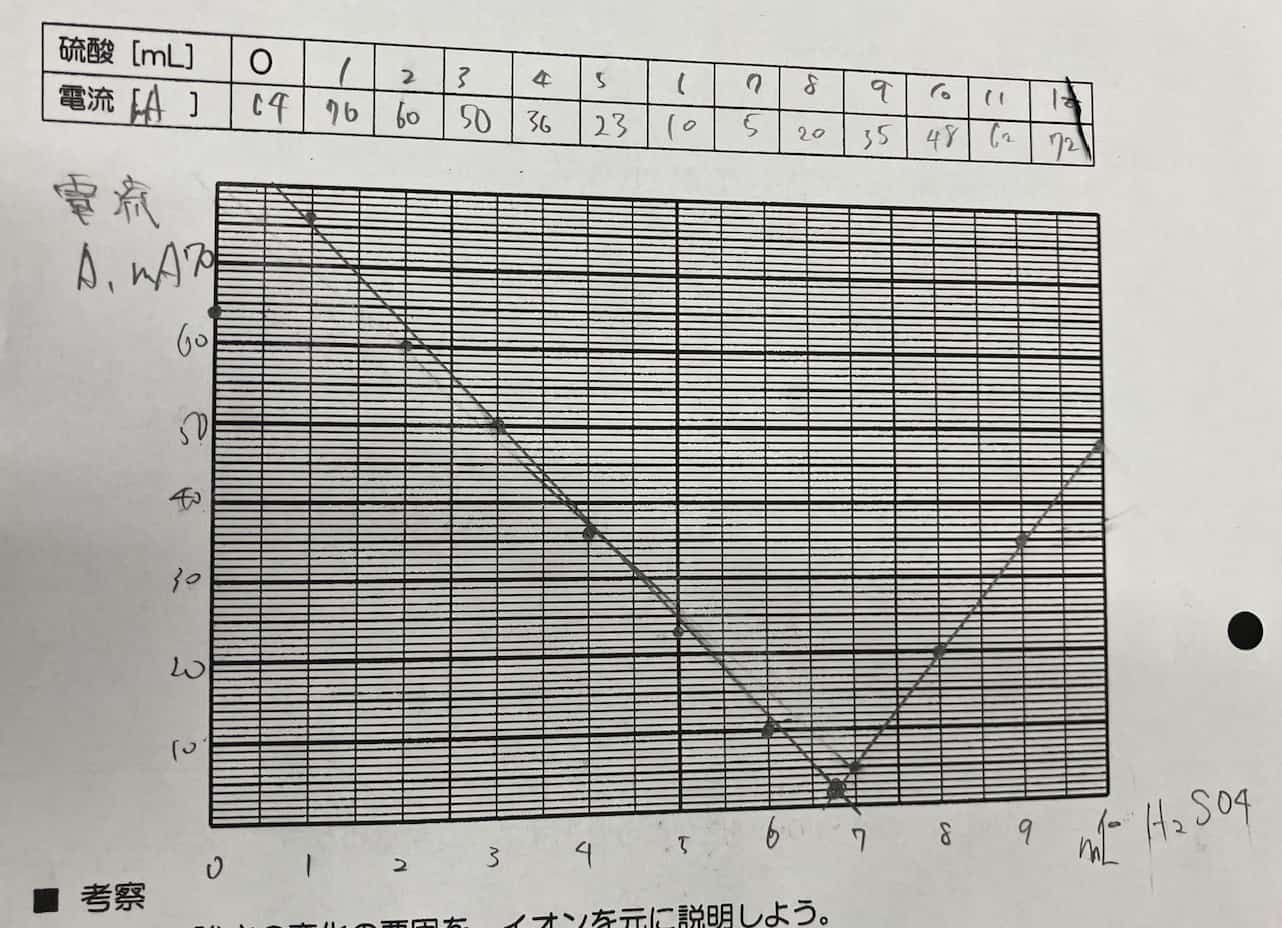
As you add sulfuric acid, the current steadily drops until it hits nearly zero at one specific point. But keep adding acid beyond that point, and the current starts to climb again!
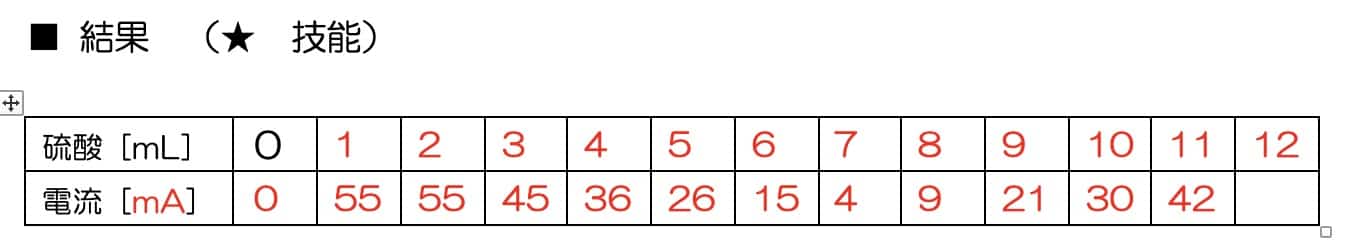
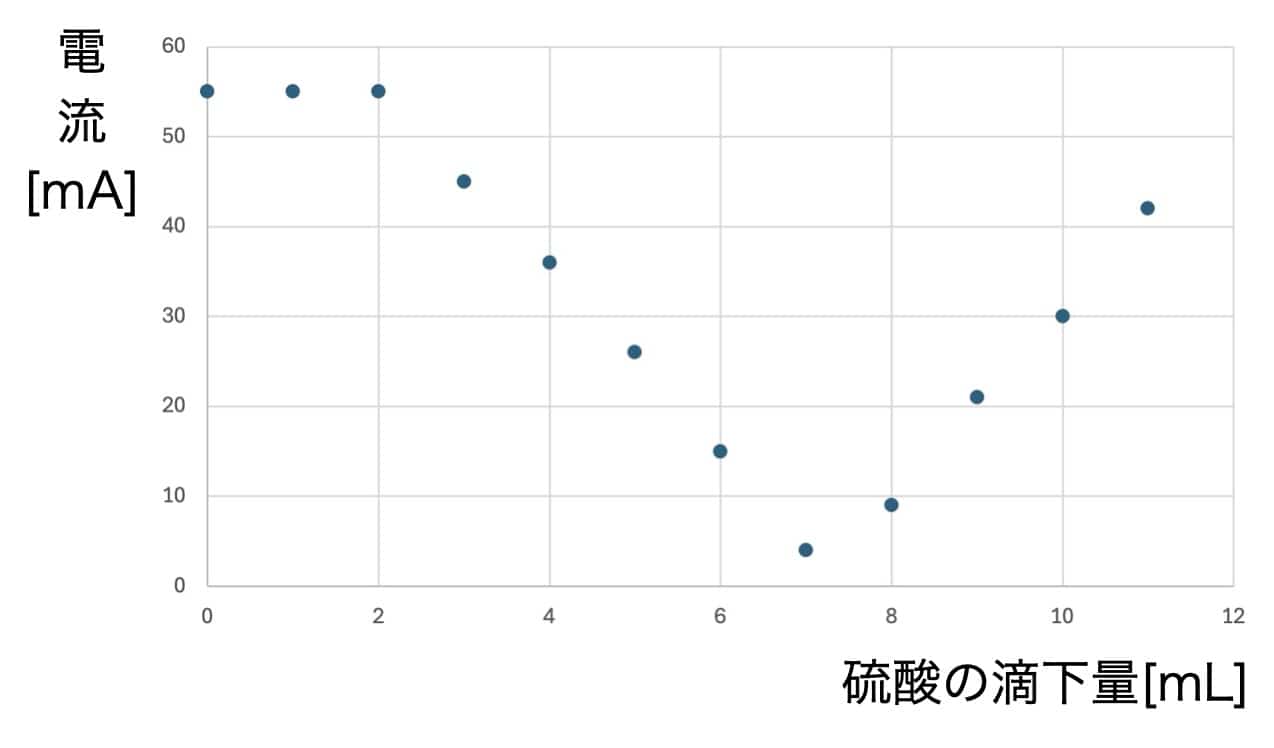
Why do we get this perfect V-shape? The secret lies in the formation of an “insoluble salt.” When barium hydroxide and sulfuric acid meet, they react to form barium sulfate—a white precipitate that does not dissolve in water.
The ions that were busy carrying electricity through the liquid bond together to form solid particles (the precipitate) and sink to the bottom. Since the “delivery drivers” of the current (the ions) are gone, the electricity stops flowing. However, as you continue to add more sulfuric acid, you introduce an excess of new ions, which allows the current to flow once again.
In a reaction between hydrochloric acid and sodium hydroxide, the resulting salt is sodium chloride (table salt). Because sodium chloride stays dissolved in water as ions, this “zero-current” phenomenon doesn’t happen. The fact that the salt is insoluble is the key to this experiment.
Wrapping Up: Science is Found in the “Change”
Watching the current steadily march toward zero is a thrill no matter how many times I see it. Rather than just reading “ions decrease” in a textbook, seeing the ammeter needle stop with your own eyes gives you a real sense that “something is happening in the invisible microscopic world!” This experiment is a perfect blend of chemical beauty and data-driven discovery. I hope you’ll try it out and experience the scientific drama hidden within the valley of that V-shaped graph!
Inquiries and Requests
Bringing the wonder and fun of science closer to you! I’ve compiled many fun experiments you can try at home and tips for success. Feel free to explore more!
My “Science Notebook” content is now a book! Details here.
Learn more about the author, Ken Kuwako, here.
For various requests (writing, lectures, science workshops, TV supervision/appearances, etc.), click here. – Stay updated on X (Twitter)!
![]() Watch experiment videos on the Science Material Channel!
Watch experiment videos on the Science Material Channel!
2月のイチオシ実験!梱包材で遊ぼう!
- 静電気の時期になってきました。子供と一緒に梱包材で盛り上がろう!→ やめられなくなる!静電気実験20
 体中に梱包材をはりつけてみよう!
体中に梱包材をはりつけてみよう!
テレビ番組等・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師等・ショー・その他お知らせ
- 2/20(金)「生徒の進学希望実現支援事業」研究授業@福井県立若狭高等学校 講師
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) サイエンスショー予定
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。


