Just Stack It and Electricity Flows! The Burger-Style Voltaic Battery and the Drama of Redox Reactions
I’m Ken Kuwako, your science trainer. Every day is an experiment!
A few drops of liquid, and a stationary propeller suddenly springs to life, spinning with incredible energy. It feels like magic, but this is the very foundation of the “battery”—a discovery by Italian physicist Alessandro Volta in the late 18th century that forever changed human history. Today, I want to take you through the fascinating drama of energy created by metals and liquids, featuring a Voltaic pile experiment we conducted at the SEPUP research group.
Recreating the World’s First Battery: The Hamburger Style Pile
For this experiment, we don’t need any fancy equipment. The stars of the show are a copper plate, a zinc plate, and a piece of filter paper to soak up the liquid. With these simple materials, you can generate electricity surprisingly easily. We used a dedicated experiment kit provided by Narika. First, we prepare these neatly cut metal plates.
First, we prepare these neatly cut metal plates. The method is quite unique. We stack the copper plate, the filter paper, and the zinc plate in that order, making it look just like a hamburger. Then, we use a pipette to add a few drops of 10% diluted hydrochloric acid, and then…https://youtu.be/Og9WMH9wJssAs you can see, the propeller connected to the motor starts spinning round and round!
The method is quite unique. We stack the copper plate, the filter paper, and the zinc plate in that order, making it look just like a hamburger. Then, we use a pipette to add a few drops of 10% diluted hydrochloric acid, and then…https://youtu.be/Og9WMH9wJssAs you can see, the propeller connected to the motor starts spinning round and round!
The Weak Point of the Voltaic Pile: Polarization
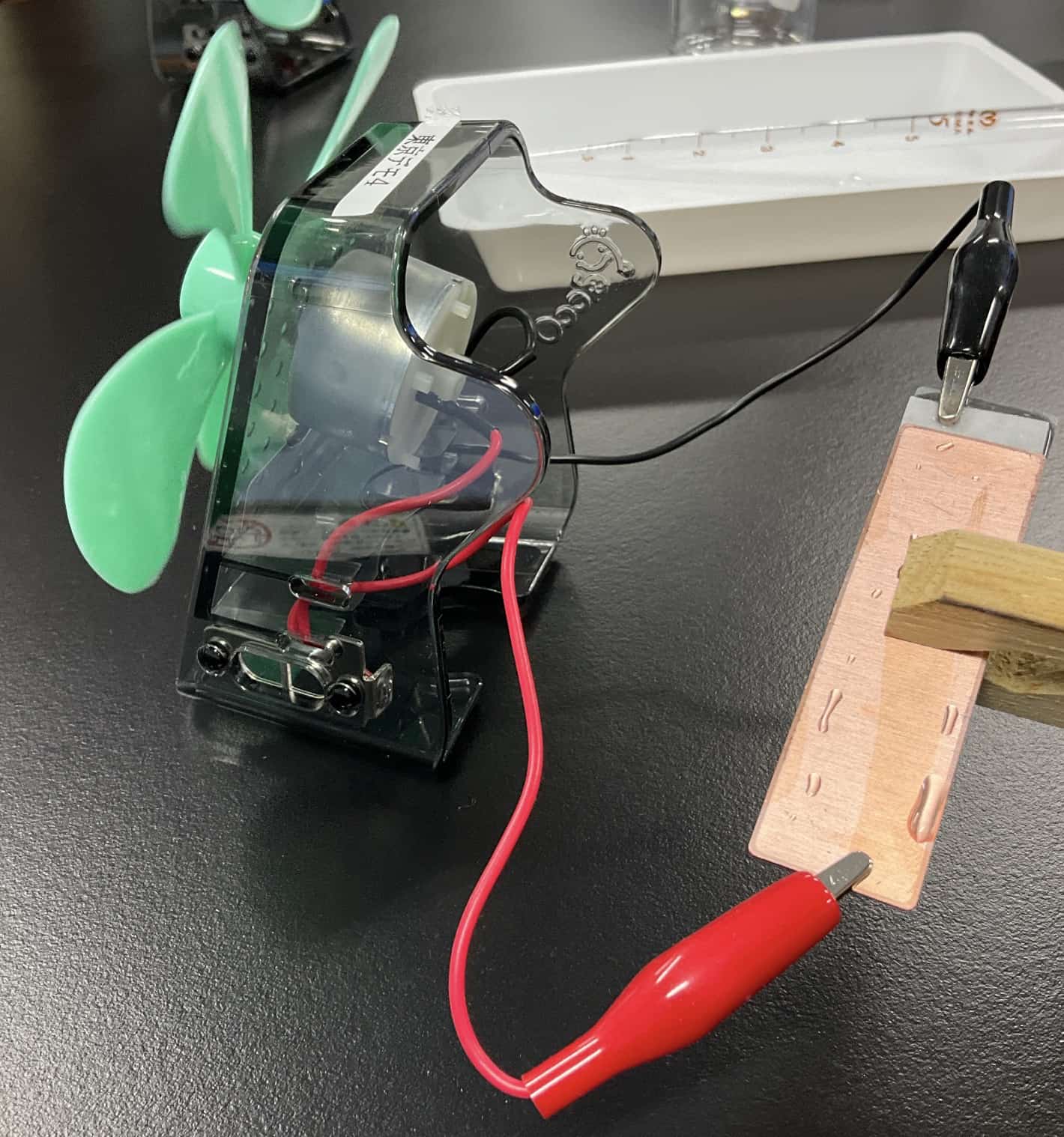 While the propeller is spinning, the original Voltaic pile actually has a major flaw. After a short while, the rotation begins to slow down. When we measure the voltage at this point, it’s only about 0.8V. The reason becomes clear if you look closely at the surface of the copper plate. It’s covered in fizzing hydrogen bubbles that end up blanketing the entire plate. This phenomenon is called polarization. The hydrogen blocks the flow of electricity, causing the battery’s life to drain almost immediately. Modern batteries, like the Daniell cell, are designed to prevent this, but it’s an unavoidable hurdle for the classic Voltaic pile.
While the propeller is spinning, the original Voltaic pile actually has a major flaw. After a short while, the rotation begins to slow down. When we measure the voltage at this point, it’s only about 0.8V. The reason becomes clear if you look closely at the surface of the copper plate. It’s covered in fizzing hydrogen bubbles that end up blanketing the entire plate. This phenomenon is called polarization. The hydrogen blocks the flow of electricity, causing the battery’s life to drain almost immediately. Modern batteries, like the Daniell cell, are designed to prevent this, but it’s an unavoidable hurdle for the classic Voltaic pile.
Burning the Copper? A Surprising Power-Up Trick
This is where science experiments get really fun. You can dramatically revive a dying propeller with one simple “extra step.” The trick is to heat the copper plate with a gas burner until an oxide film forms on the surface. When we repeat the experiment using this blackened, seemingly “burnt” copper plate, the propeller spins even faster than before! The electromotive force jumps up to about 1.1V.Why does a “dirty” copper plate produce more power? It’s because the copper oxide on the surface helps the process of receiving electrons.
When we repeat the experiment using this blackened, seemingly “burnt” copper plate, the propeller spins even faster than before! The electromotive force jumps up to about 1.1V.Why does a “dirty” copper plate produce more power? It’s because the copper oxide on the surface helps the process of receiving electrons.
\[\text{(Electrodo positivo):} \text{Cu}\_2\text{O} + 2\text{H}^+ + 2\text{e}^- \rightarrow 2\text{Cu} + \text{H}\_2\text{O}\]
This reaction suppresses the formation of hydrogen bubbles, giving the voltage a significant boost. When the voltage eventually drops again and you remove the plate, the black copper oxide has vanished, leaving the metal shiny and bright once more. Being able to see the reaction “finishing” through this visual change is part of the charm of this experiment.
Learning History Through Trial and Error
Compared to modern batteries, the Voltaic pile is certainly not high-performance. However, it is an incredible educational tool that allows us to experience the grand discovery that combining different metals can extract electricity. The more you dig into it—like learning how the copper plate acts as a catalyst for hydrogen—the deeper the world of science becomes. The Voltaic pile is finicky and unpredictable, but that’s exactly why it inspires us to ask, “How can I make it run longer?” I encourage you to see the first step of battery history with your own eyes.https://phys-edu.net/wp/?p=49892
Inquiries and Requests
Let’s make the wonders of science a part of your daily life! I’ve put together plenty of fun experiments you can try at home along with helpful tips. Take a look around!My “Science Notebook” content is now a book! Details here.Learn more about the author, Ken Kuwako, here.For various requests (writing, lectures, workshops, TV supervision, appearances, etc.), click here.– Get the latest updates on X (formerly Twitter)!
![]() Check out my experiment videos on the Science Material Channel!
Check out my experiment videos on the Science Material Channel!
2月のイチオシ実験!梱包材で遊ぼう!
- 静電気の時期になってきました。子供と一緒に梱包材で盛り上がろう!→ やめられなくなる!静電気実験20
 体中に梱包材をはりつけてみよう!
体中に梱包材をはりつけてみよう!
テレビ番組等・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師等・ショー・その他お知らせ
- 2/20(金)「生徒の進学希望実現支援事業」研究授業@福井県立若狭高等学校 講師
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) サイエンスショー予定
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。


