Learn How Engines Work with a Shocking “Pop!” — The Secret of Alcohol Explosion Experiments (First Law of Thermodynamics)
I am Ken Kuwako, your Science Trainer. Every day is an experiment.
The “Pop!” That Opens the Door to Science: A Safe At-Home Thermodynamics Experiment
【This article is also available on Radio!】
There is something instinctively thrilling about seeing something launch right before your eyes. Have you ever looked at a simple everyday object and wondered, “How does that actually work?”
Today, I want to share a thrilling yet safe science experiment to spark your intellectual curiosity. The star of the show? Hand sanitizer (rubbing alcohol), something found in almost every household. It is the magic key to unlocking a powerful, invisible force.
The word “explosion” might sound a bit intense, but don’t worry. This experiment simply harnesses the power of a tiny amount of alcohol turning into gas and rapidly expanding. You will get to hear, see, and feel “gas doing work” in real-time. This phenomenon is the gateway to the profound world of thermodynamics—the same principles that power car engines and launch rockets into space.
Join me as we experience this surprising “Pop!” and rediscover the joy of science!
This experiment isn’t just a great way for middle schoolers to understand the difference between “combustion” and “explosions”; it is also the perfect visual aid for grasping the “First Law of Thermodynamics,” which is often a hurdle in high school physics.
The Science Recipe: Try the Alcohol Explosion Experiment!
The heart of this experiment lies in the fact that burning a small amount of alcohol instantly heats and expands the air inside a sealed can. This trapped energy, looking for an escape, provides the power to launch a paper cup lid high into the air.
What You Will Need
- Paper Cup: Acts as your lid (the projectile). A standard size is perfect.
- Aluminum Can: An empty soda can works best. One with a flat bottom is more stable.
- Can Opener: To remove the top of the aluminum can.
- Awl (or a sharp tool): To poke a small ignition hole in the bottom of the can.
- Rubbing Alcohol: Ethanol with a concentration of 70% or higher. Spray types are highly recommended as they mist easily.
- Lighter or Multi-purpose Lighter: For ignition.
- Safety Gear: Always have a wet cloth and a bucket of water ready.
Experimental Procedure: Safety First!
Once you are ready, let’s start. Precision and care with each step are the secrets to both success and safety.
- Cut the top off the can Use the can opener to completely remove the top (the drinking side) of the aluminum can. Be careful not to cut your hands on the sharp edges. Wash and dry the inside thoroughly.
- Create the ignition hole Using the awl, poke a tiny hole in the center of the bottom of the can. This will be your “fire gateway.” Work on a stable surface and be careful not to slip.
- Add the alcohol (Most Important Step!) Spray just 2 pumps or add 2-3 drops of alcohol into the can. NEVER add more than this. Too much alcohol can cause a large flame to linger after the explosion, which is dangerous. This “tiny amount” is what creates the perfect scientific reaction.

- Vaporize and mix Place the paper cup lightly on top of the can. Give the can a few gentle shakes to help the alcohol evaporate and distribute the vapor throughout the can.

- The Moment of Ignition! Double-check that your wet cloth and water bucket are within reach. Bring the lighter flame close to the hole at the bottom.

Results: The “Pop” is Thermodynamics in Action
https://youtu.be/mTNW7m13Eyc
The moment you bring the flame close, you’ll hear a satisfying “Pop!” and the paper cup will soar! This happens because the alcohol vapor burns instantly, causing the air temperature inside to skyrocket. This hot air wants to expand aggressively, and that force pushes the paper cup toward the ceiling.
This is a real-world experience of the First Law of Thermodynamics. The “chemical energy” of the alcohol converted into “thermal energy” via combustion. That heat then performed “work” by pushing the gas, which finally became the “kinetic energy” that launched the cup. Energy is constantly shifting forms, dancing right before your eyes.
We captured this heat movement with a thermographic camera. Look at these incredible results:
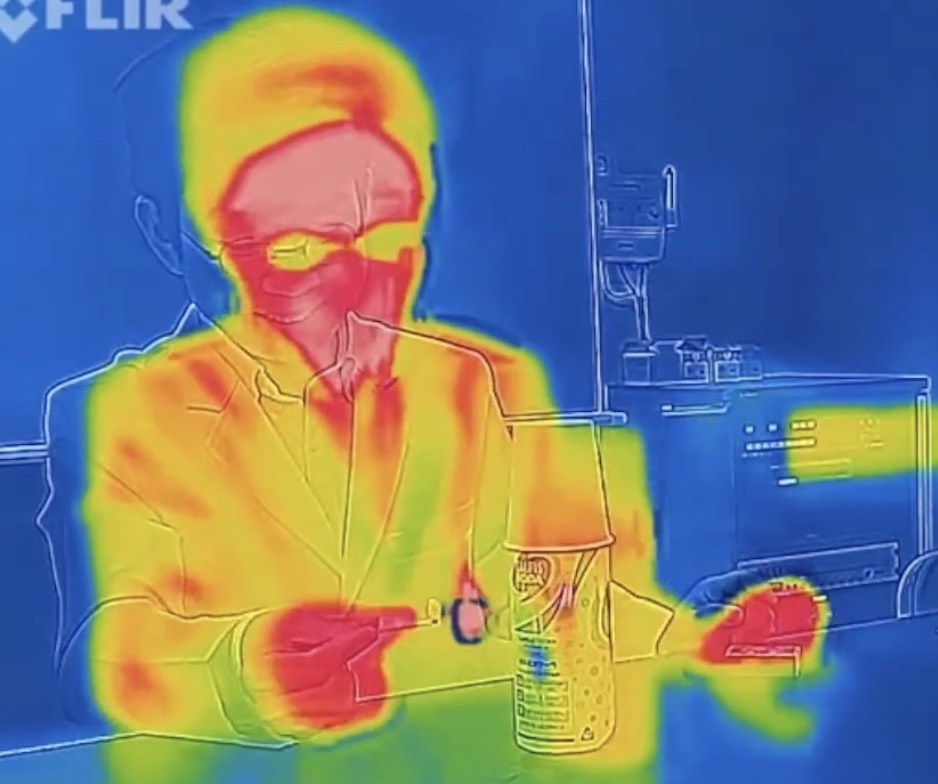 Just before ignition.
Just before ignition.
 At the moment of the “Pop!”, the internal temperature spikes into the red!
At the moment of the “Pop!”, the internal temperature spikes into the red!
 【WARNING!】 Never look directly into the can from above during the experiment. It is extremely dangerous.
【WARNING!】 Never look directly into the can from above during the experiment. It is extremely dangerous.
【Golden Rules for a Safe Experiment】
- No Overdosing!: Limit the alcohol to 2 pumps. Being greedy with the amount can lead to a sustained fire inside the can. If a flame persists, cover it with a wet cloth to cut off the oxygen and extinguish it calmly.
- Adult Supervision Required: This experiment deals with the “power” of science. Students should always perform this with a parent, guardian, or teacher in a clear area away from flammable materials.
- Ventilation is Key: The air after the experiment contains combustion gases. Open a window and ensure good airflow.
For those who want to dive deeper into these thermodynamic wonders or revisit high school physics, my book “High School Physics Review for Adults” offers even more fun and detailed explanations. Give it a read, and you’ll find that the invisible flow of energy feels much more familiar.
A dynamic world of science is hiding in your kitchen cabinets. Are you ready to be amazed by the “work of gas” taught by just a few drops of alcohol? Stay safe and give it a try!
Inquiries and Requests
Making the wonders of science more accessible! I’ve compiled many fun experiments you can do at home. Feel free to explore!
- Learn more about the author, Ken Kuwako, here
- For requests (writing, lectures, workshops, TV supervision/appearances), click here
- Follow me on X (formerly Twitter) for updates!
![]() Check out my experiment videos on the Science Material Channel!
Check out my experiment videos on the Science Material Channel!
2月のイチオシ実験!梱包材で遊ぼう!
- 静電気の時期になってきました。子供と一緒に梱包材で盛り上がろう!→ やめられなくなる!静電気実験20
 体中に梱包材をはりつけてみよう!
体中に梱包材をはりつけてみよう!
テレビ番組等・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師等・ショー・その他お知らせ
- 2/20(金)「生徒の進学希望実現支援事業」研究授業@福井県立若狭高等学校 講師
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) サイエンスショー予定
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。




