Escape the Chemistry Labyrinth: Master Mole Calculations with One Simple Flowchart!
I’m Ken Kuwako, your science trainer. Every day is an experiment!
“I started learning chemistry, but the moment the mole (mol) appeared, my mind went totally blank…”Have you ever felt that way? The mole is the first major wall students hit in high school chemistry. This unit, which involves counting invisible, microscopic particles, is often the entrance to a “chemistry labyrinth” for many.But don’t worry! Once you understand its true nature, the mole is actually an incredibly helpful bridge. Today, I’ll explain everything from the concrete image of a mole to a “magic flowchart” that makes calculations easy for anyone!
The Mole: A “Giant Set”
One mole (mol) refers to a collection of particles numbering \[6.0 \times 10^{23}\] (that’s a 6 followed by 23 zeros!). Just as a dozen pencils is 12 and a pack of eggs is 10, the world of chemistry uses this massive “set” as a unit to handle atoms and molecules that are otherwise too tiny to count.Let’s take a look at what 1 mole actually looks like.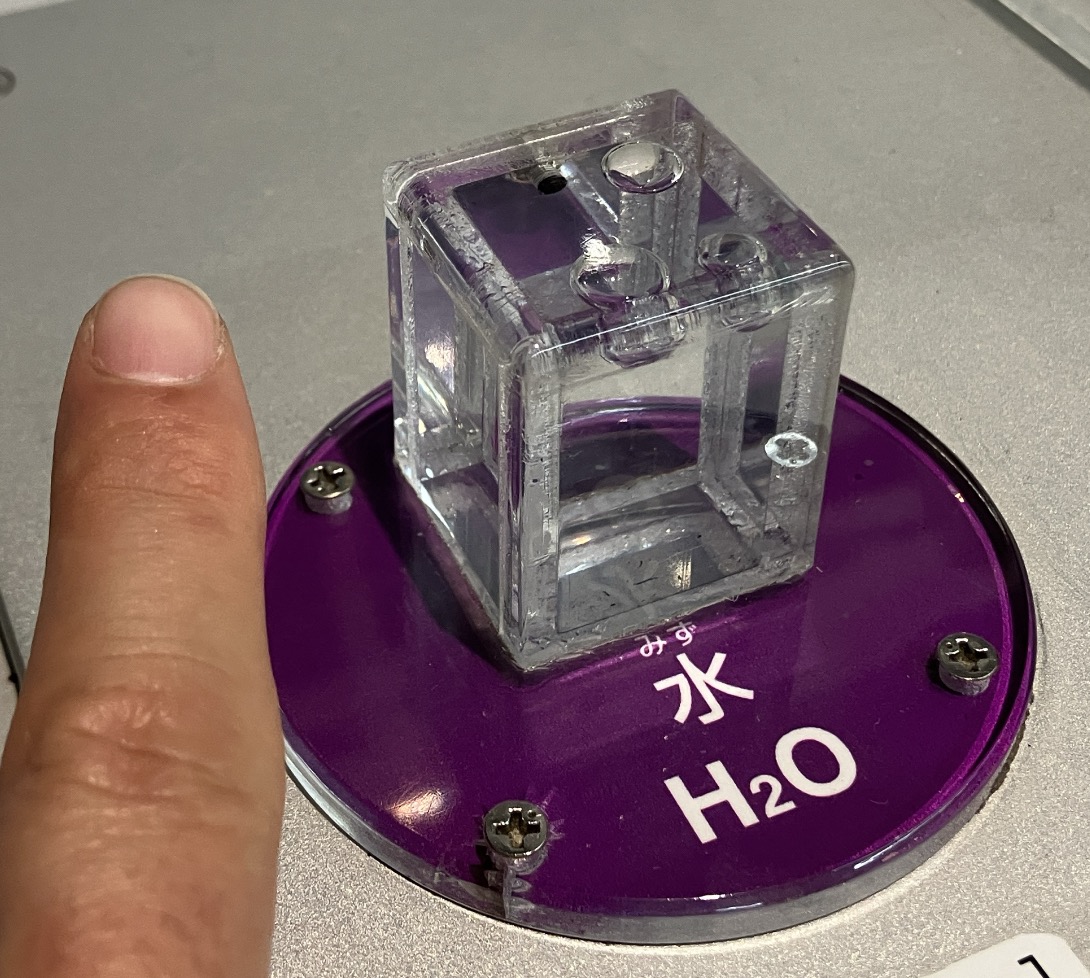 This is a model of “1 mole of water” displayed at the National Museum of Nature and Science. When 6.0 times 10^{23} water molecules gather, they weigh 18g. In terms of volume, it’s a small cube about the size of a knuckle on your index finger. You might think, “That’s surprisingly small!”However, it’s a completely different story when it comes to “air.”
This is a model of “1 mole of water” displayed at the National Museum of Nature and Science. When 6.0 times 10^{23} water molecules gather, they weigh 18g. In terms of volume, it’s a small cube about the size of a knuckle on your index finger. You might think, “That’s surprisingly small!”However, it’s a completely different story when it comes to “air.” Look at that! It’s much larger. For gases at standard temperature and pressure (STP), 1 mole takes up a volume of 22.4L (liters), regardless of the type of gas. This comparison of “1 mole” clearly shows how the distance between molecules differs drastically between liquids and gases. By the way, the amount of carbon in a pencil lead is said to be about 0.1 moles.
Look at that! It’s much larger. For gases at standard temperature and pressure (STP), 1 mole takes up a volume of 22.4L (liters), regardless of the type of gas. This comparison of “1 mole” clearly shows how the distance between molecules differs drastically between liquids and gases. By the way, the amount of carbon in a pencil lead is said to be about 0.1 moles.
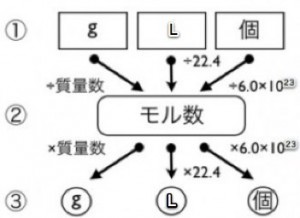
The “Mole Flowchart”: Never Get Lost Again
The reason people struggle with calculations is that they lose sight of the path—the “from what to what” logic. To help with this, I’d like to share the flowchart I always use when teaching.
The key point of this diagram is that “All roads lead to the Mole.” If you want to change mass (g) into volume (L), it’s hard to convert them directly. However, if you pass through the “Mole Station” first, the calculation becomes smooth and simple.
Let’s Practice! Master the Trick with an Example
Example: How many liters (L) does 36g of water vapor occupy at STP?Let’s solve this by applying the flowchart!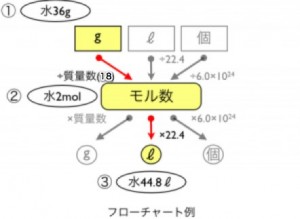
Step 1: Mass (g) → Mole (mol)First, convert the given 36g of water into moles. The molecular weight of water ($H_2O$) is $H(1) \times 2 + O(16) = 18$.$36g \div 18g/mol = 2 \text{ moles}$.2.
Step 2: Mole (mol) → Volume (L)Next, calculate how many liters 2 moles of water vapor will occupy. Since 1 mole of any gas is 22.4L at STP:$2 \text{ moles} \times 22.4L/mol = 44.8L$!See? By following the rule of “converting to moles first before heading to your destination (L or number of particles),” even complex-looking calculations can be solved like a puzzle.It might feel unfamiliar at first, but try solving some problems with this flowchart by your side. You’ll soon experience that “Aha!” moment. Whenever you’re stuck, just come back to this diagram!
Inquiries and Requests
Bringing the wonder and fun of science closer to you! I’ve put together many fun experiments you can do at home and easy-to-understand tips. Feel free to explore!My “Science Notebook” content is now a book! Details hereLearn more about Ken Kuwako hereFor work requests (writing, lectures, workshops, TV supervision, etc.), please contact me here– Follow me on X (formerly Twitter) for updates!
![]() Watch experiment videos on my Science Material Channel!
Watch experiment videos on my Science Material Channel!
2月のイチオシ実験!梱包材で遊ぼう!
- 静電気の時期になってきました。子供と一緒に梱包材で盛り上がろう!→ やめられなくなる!静電気実験20
 体中に梱包材をはりつけてみよう!
体中に梱包材をはりつけてみよう!
テレビ番組等・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師等・ショー・その他お知らせ
- 2/20(金)「生徒の進学希望実現支援事業」研究授業@福井県立若狭高等学校 講師
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) サイエンスショー予定
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。