The Science Behind the Jiggle! The Magic of Slime—and Electrifying “Electric Slime”
I am Ken Kuwako, Science Trainer. Every day is an experiment.
“It is slimy, cold, and feels so good!”
This mysterious texture is exactly why everyone from elementary to junior high school students gets hooked on this magical substance. It is called slime. While it is a classic science lab experiment, it is a waste to just end it with “that was fun!”
In reality, slime is an entry point into the world of polymers, packed with the wonders of chemistry. This time, I will explain everything from the dramatic mechanism of how slime solidifies to an amazing applied experiment where we turn slime into a pathway for electricity, all from a scientific perspective.

How to Make It
First, let’s start with basic slime making. Mastering the golden ratio is the secret to success.
Ingredients for making slime:
- Laundry glue (PVA) 50g
- Hot water around 60°C 50g (or water). The key point is mixing the laundry glue and water 1:1.
- Borax 20g
- Water 200g
- 3 paper cups, a scale, and disposable chopsticks
- Paint or food coloring
Laundry glue (PVA) and Borax can be purchased at drugstores. You can often find them in the laundry detergent section, and Borax is frequently located in the skin medication section (near disinfectants).
Laundry Glue

Borax 
Additionally, Borax is originally used for convenient purposes like these:
Disinfection and Deodorization: Deodorizing trash cans, repelling mites from carpets, cleaning toilets, etc.
Laundry: Since it has the effect of softening water, adding a small amount with detergent increases cleaning power.
[Important Precautions] Although Borax is a naturally derived substance, it is also toxic.
Do not put it in your mouth: Manage it strictly so that small children or pets do not accidentally ingest it.
Do not touch open wounds: Since it is easily absorbed through the skin, wear gloves if your hands are irritated.
Experimental Procedure
Make a saturated Borax aqueous solution. Put 200g of water in a paper cup, add 20g of Borax, and mix well with chopsticks. Some undissolved residue will collect at the bottom, but do not worry about it. We will use the clear supernatant liquid.
Prepare 50g of hot water around 60°C in another paper cup (hot water makes the reaction smoother, but water is also possible). Here, add your favorite food coloring or paint to create your own unique color!
Measure out 50g of laundry glue (PVA) in yet another paper cup.

While pouring the PVA into the hot water, stir thoroughly until there is no unevenness.
Add 20g of the Borax solution supernatant made in step 1 to the mixed liquid little by little while stirring.

After stirring for about 30 seconds, the resistance will gradually become heavier, and it will clump together. Your slime is finished!


The Mechanism of Slime Solidification: Microscopic Bridging
“Thick laundry glue turns into jiggly slime the moment you add Borax water.” Behind that change, a dynamic drama of molecules holding hands is taking place. In chemistry, this is called a cross-linking reaction.
1. The True Identity of Laundry Glue is a “Long Chain”
The protagonist of slime, PVA (Polyvinyl Alcohol), is a polymer shaped like a long chain made of many small molecules connected together. In water, these long chains float around separately, so they can flow freely as a liquid.
2. Borax Becomes the “Bridge Builder”
When “borate ions” from dissolved Borax are added, the situation changes completely. The borate ions wedge themselves between the separately moving PVA chains and connect them together as if building a bridge.
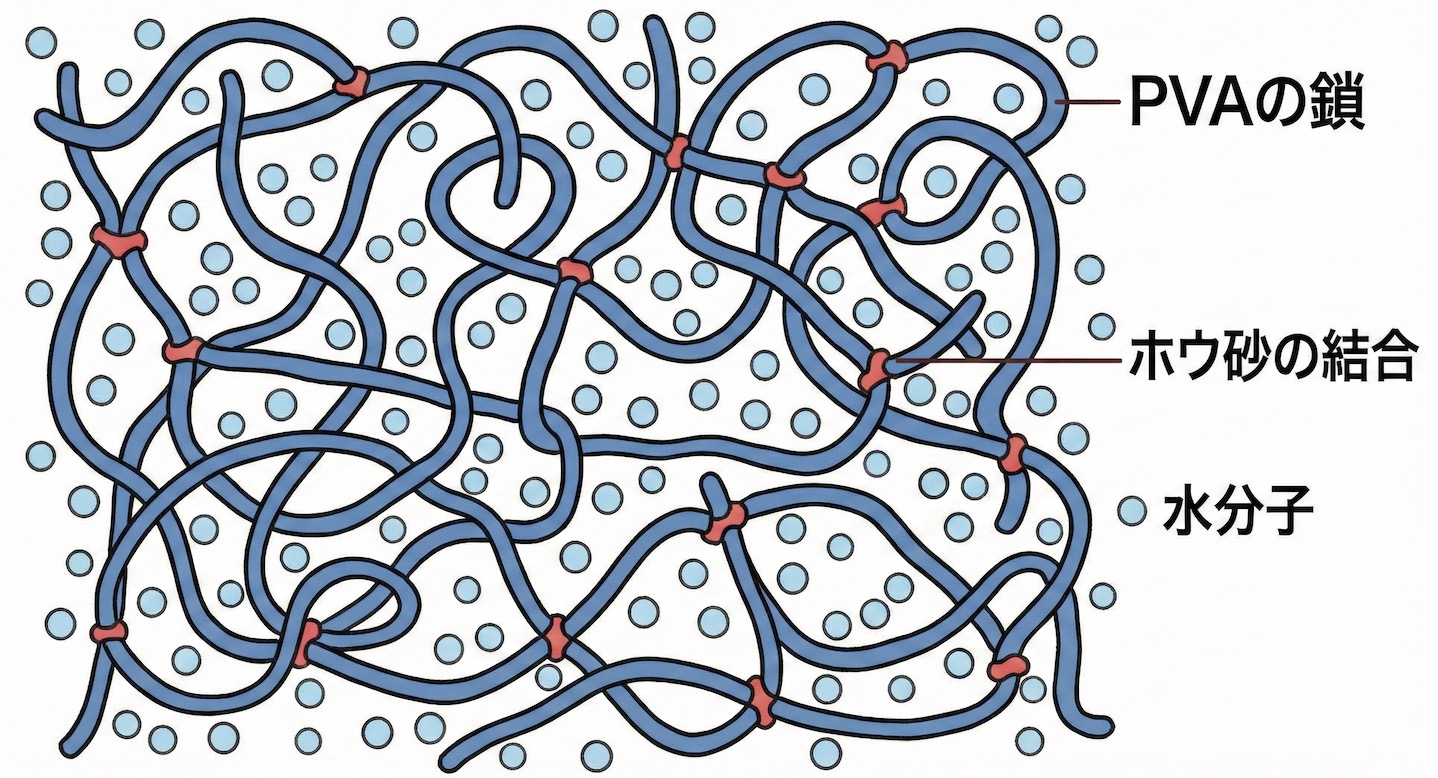
PVA: Long string-like molecules
Borate Ions: Act like clips that snap the strings together
3. Trapping Water to Become “Jiggly”
When the chains connect in a network, they trap many water molecules in the gaps. The glue that was a liquid has its movement restricted by the network structure. However, because it contains plenty of water inside, it does not become a hard solid. This intermediate state between liquid and solid (a gel) is exactly what slime is!
Surprising Application! The Electric Slime Experiment
Actually, slime can also be a star in the world of electricity as a movable conductor that can change shape freely! I will introduce an electric slime experiment that I actually tried and was moved by. Let’s take a look at a new world of play where physics and chemistry merge.
Divide the slime into two lumps and try inserting battery terminals into one and LED legs into the other. Then… surprisingly, the LED lights up instantly! (Note: If you connect the battery and LED directly, the voltage is too strong and will break the LED. Be sure to sandwich the slime in between.)

Assemble a jiggly circuit.

Switch on! It lit up brilliantly.
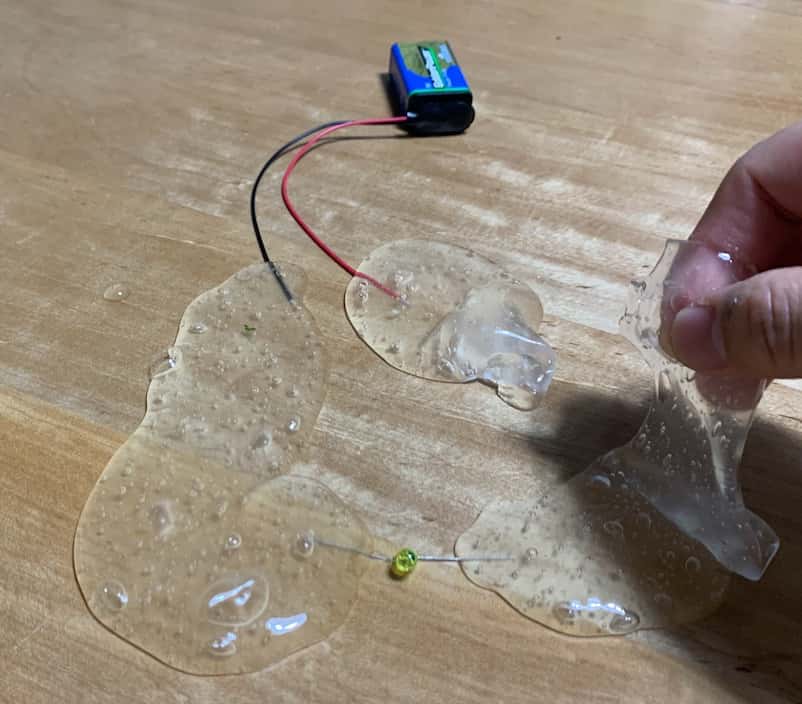
It turns off when you pull the slime apart. It is exactly like a switch.
If you make the room a little dark, the light leaking through the slime is dreamy and very beautiful.

Why Does Electricity Flow Through Slime?
Actually, the Borax dissolved in the slime is the key. When Borax dissolves in water, it separates into particles called ions that carry electricity. Because these ions can move around freely inside the slime, they can conduct electricity just like a metal wire.
What is even more interesting is that the LED gets dimmer when you stretch the slime long, and it gets brighter when you make it short and thick. This is the perfect chance to learn the basics of physics—that electrical resistance changes depending on the length and thickness of the path electricity takes—all while touching it with your hands!
It is a waste to just end it with making and touching slime. Please try experiencing this slime as an electric circuit.
Contact and Request Information
Bringing the wonders and fun of science closer to you! I have put together fun science experiments you can do at home and tips for them in an easy-to-understand way. Please try searching for various things! ・The content of the Science Notebook is now a book. See here for details. ・See here for information about the operator, Ken Kuwako. ・See here for various requests (writing, lectures, science classes, TV supervision, appearances, etc.). ・Article updates are being distributed on X!
![]() The Science Notebook Channel is distributing experiment videos!
The Science Notebook Channel is distributing experiment videos!