Boiling Without Heat?! A Mind-Blowing “Vacuum Boiling” Experiment Using Just a Syringe (Phase Changes)
I’m Ken Kuwako, your Science Trainer. Life is one big experiment!
“Water only boils when it hits 212°F (100°C)”… Do you find yourself believing that’s an absolute rule? Actually, there’s a magical way to make warm water burst into a vigorous boil in an instant—without using any heat at all.
Today, I want to introduce you to the surprising “Vacuum Boiling Experiment” that you can try with just a single syringe. Let’s watch together as “states of matter”—something that usually stays tucked away in textbooks—dynamically transforms right before your eyes.
The Magic Syringe: Boiling Water Without Changing the Temperature
In science class, we learn about “phase changes,” where water turns into ice or steam depending on the temperature. However, you can also dramatically change the state of a substance by changing the “pressure.” This simple yet profound experiment was shared with me by the wonderful Mr. Y, who I’m always grateful to.
[What You’ll Need] ・A syringe (plastic medical type) ・Warm water (around 160°F / 70°C)
[Experimental Procedure]
First, prepare some warm water (about 70°C) in a beaker or cup.
Draw a small amount of the warm water into the syringe. The key is to leave plenty of room so you can pull the plunger back significantly later.
Firmly cover the tip of the syringe with your thumb to create an airtight seal.
Now, pull the plunger back as hard as you can to drop the internal pressure.

Look at that! Even though you haven’t added any heat, the water inside suddenly starts boiling violently.

What’s even more fascinating is that as soon as you release the plunger, the boiling stops instantly. This clearly demonstrates that it’s the “change in pressure,” not the temperature, causing the water to boil.
Why Rice Doesn’t Cook Well on a Mountaintop
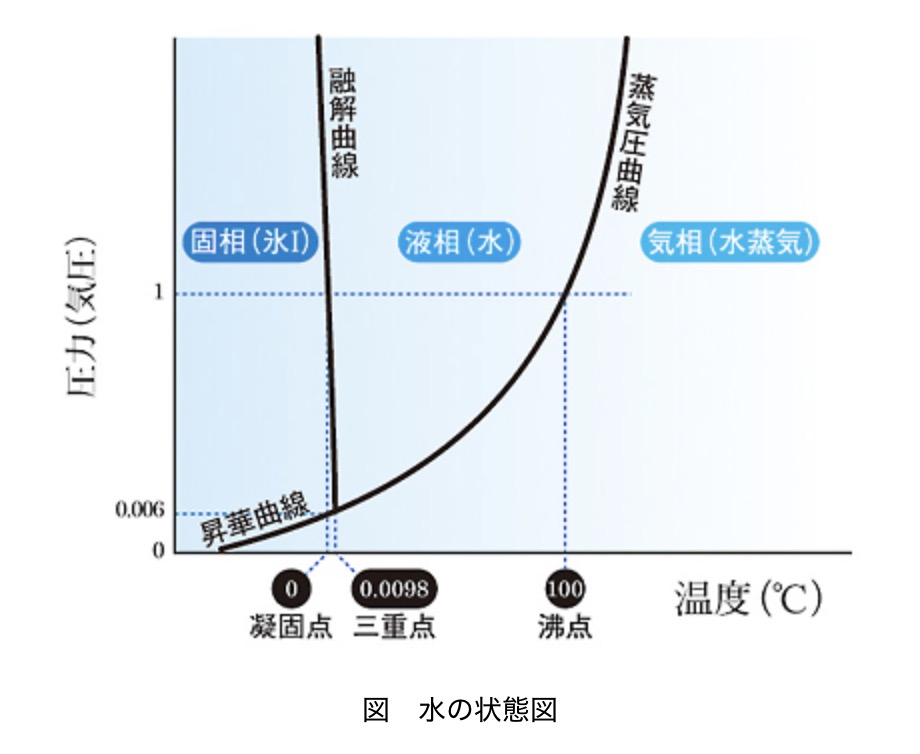
Why does lowering the pressure make water boil?
Water stays in liquid form because the weight of the surrounding air (atmospheric pressure) is pressing down on the water molecules, keeping them from escaping. Normally, at sea level (1 atmosphere), water molecules can’t overcome this downward pressure until they reach 100°C. However, when you create a near-vacuum inside the syringe, the force holding the molecules down weakens. As a result, the water molecules can break free and turn into gas even at lower temperatures, and that’s when boiling begins.
Source: Chronological Scientific Tables “Please explain the states of solids, liquids, and gases.” https://official.rikanenpyo.jp/posts/6660
This principle is closely tied to our daily lives. For example, at high altitudes like the summit of Mt. Fuji (12,388 ft), where the air pressure is only about two-thirds of that at sea level, water boils at around 175°F to 195°F (80-90°C). This is why if you try to cook rice in a regular pot up there, you’ll end up with crunchy, undercooked grains!
Ever noticed how bags of chips puff up when you go to high altitudes?

The best part of science is that you can recreate the environment of a mountain peak using just a simple syringe. Give it a try and see how cool the water can be while still making it boil!
Inquiries and Requests
Bringing the wonder and fun of science closer to you! I’ve compiled various fun experiments you can do at home and easy-to-understand tips. Feel free to explore more! ・The “Science Notebook” content is now a book! Details here ・Learn more about the administrator, Ken Kuwako, here ・For various requests (writing, lectures, science workshops, TV supervision, appearances, etc.), click here ・Get the latest updates on X (Twitter)!
![]() Watch experiment videos on the Science Material Channel!
Watch experiment videos on the Science Material Channel!

