Eco-Friendly, Simple, and Easy to Clean Up! Build a “Hamburger-Style” Daniell Cell Just by Stacking
I’m Science Trainer Ken Kuwako. Every day is an experiment!
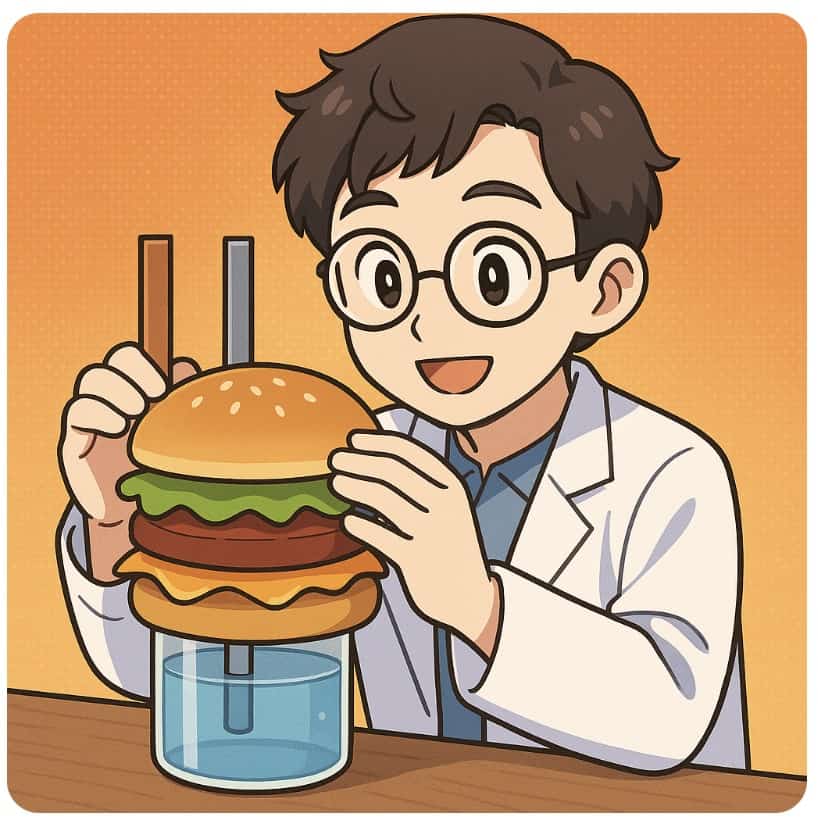
The Daniel Cell is a classic and essential topic for understanding how batteries work. However, I know many teachers struggle with the lengthy preparation and the hassle of disposing of chemical waste.
That’s why today, I want to introduce the Hamburger-style Daniel Cell! True to its unique name, this is a revolutionary, simplified version of the Daniel cell that you build by layering materials just like a hamburger. Beyond its fun appearance, its biggest draw is how eco-friendly it is—it produces significantly less waste than a standard setup. Plus, because it uses common materials, it dramatically reduces the burden of lesson prep.
Of course, being a simplified version, you can’t see the solution changing directly. But don’t worry! By combining this with a standard Daniel Cell for demonstration, you can get the best of both worlds and truly deepen your students’ understanding.
For example, you can use the standard cell to visually show ion movement and metal dissolving/depositing, and then have the students get hands-on with the “Hamburger” version to experience power generation themselves. This multi-angled approach helps students grasp the core principles while having a blast. Why not try this fun, eco-friendly experiment in your next class and hear your students go, “Aha!”?
Materials and How to Make It
The secret ingredient for this Hamburger-style Daniel Cell is cellophane. Just like the porous pot or salt bridge in a standard cell, it acts as a barrier that prevents the different electrolytes from mixing while allowing ions to pass through. Amazingly, even the colored cellophane found at 100-yen stores works perfectly, keeping costs and prep time low. By using filter paper (or even kitchen towels) as a medium to hold the solutions, there’s almost no liquid waste, making cleanup a breeze.

Believe it or not, I’ve even heard you can use standard printer paper instead of cellophane! You can also substitute the zinc sulfate solution with saturated salt water if needed.
What You’ll Need:
- Zinc Sulfate Solution: Approx. 5% (Dissolve 9.8g of zinc sulfate heptahydrate in 100g of water)
- Copper Sulfate Solution: Approx. 17% (Dissolve 35.7g of copper(II) sulfate pentahydrate in 100g of water)
- 2 plastic pipettes (these are recommended), 2 Petri dishes, 1 Zinc plate, 1 Copper plate, 2 pieces of filter paper (kitchen towels work too), 1 sheet of cellophane, 1 tissue, a small propeller, an electronic melody maker (by Narika), a multimeter (this one is recommended), alligator clips, and tweezers. I bought my cellophane at a 100-yen store.
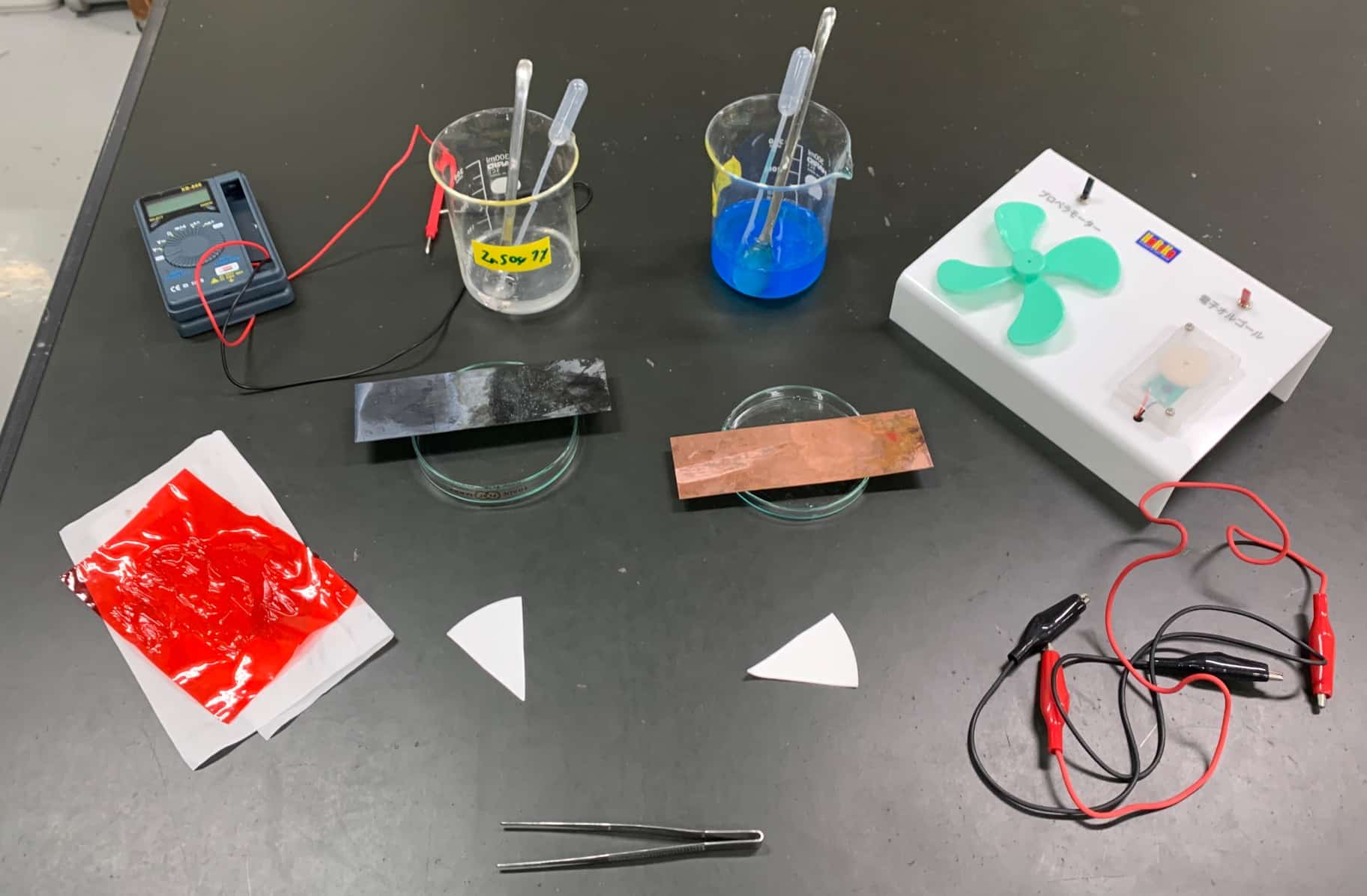
Here is everything you need.
Experimental Procedure
Now, let’s assemble the Hamburger Daniel Cell. The steps are incredibly simple—it’s just like stacking a sandwich!
Place the filter papers in the Petri dishes and use the pipettes to soak them with the copper sulfate and zinc sulfate solutions.

Place a tissue on the desk and put the copper plate on top. It’s easiest to do this at the edge of the table. Follow this stacking order:
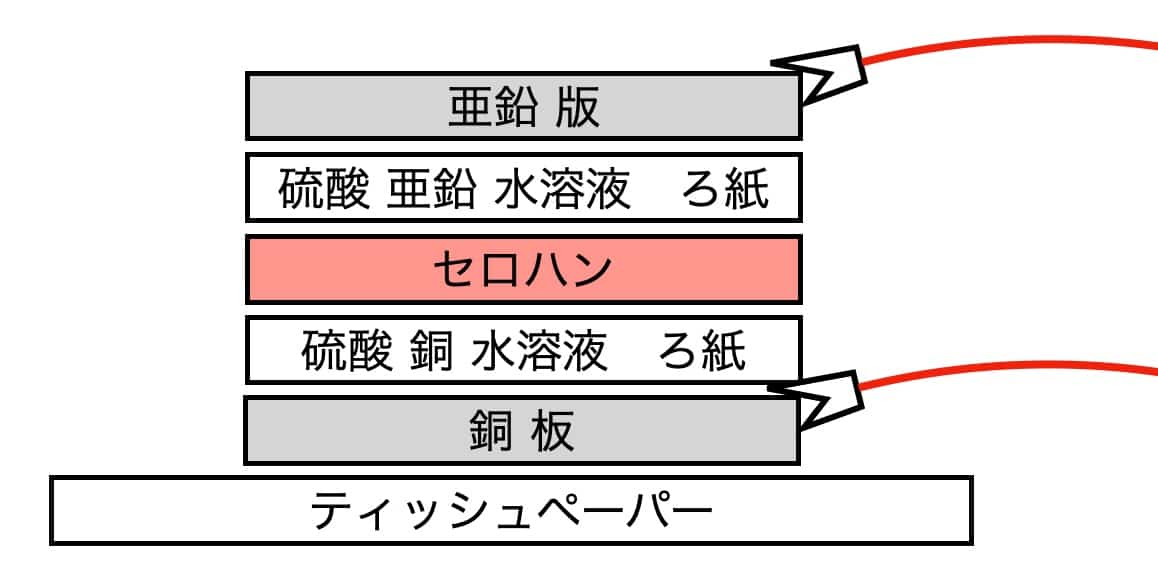
Tissue -> Copper plate -> Filter paper soaked in copper sulfate -> Cellophane.

Next, add the filter paper soaked in zinc sulfate, and finally, top it off with the zinc plate.

Be careful not to let the copper and zinc plates touch each other directly. Connect the plates to the melody maker or motor using alligator clips, then measure the voltage with your multimeter.

When I made it, the voltage was around 1V. It’s great if you can hit 1.1V!
3. To keep the current flowing, short the circuit by connecting the two clips together and leave it for about 2 minutes. Afterward, peel off the papers from each metal plate and observe any color changes.

The paper from the copper plate turned a reddish-brown color.

The paper from the zinc plate also turned a bit brown. Perhaps some copper ions passed through the cellophane?
Simply put, a Daniel Cell is a device that generates electricity by utilizing the difference in power between two metals as they try to turn into ions (dissolve in water).
How the Daniel Cell Works
Let’s look at the main players in a Daniel Cell:
- Zinc (Zn) Plate: Acts as the negative terminal (- electrode).
- Copper (Cu) Plate: Acts as the positive terminal (+ electrode).
- Zinc Sulfate Solution (ZnSO₄): The liquid the zinc plate is “dipped” in.
- Copper Sulfate Solution (CuSO₄): The liquid for the copper plate. It’s distinctively blue.
- Cellophane (or Porous Pot): The wall that keeps the two solutions from mixing instantly.
The Secret of the Flow
The flow of electricity (current) is actually the movement of tiny particles called electrons. In a Daniel Cell, there’s a “competition” going on that pushes these electrons out. It’s a contest of how much a metal wants to become an ion and dissolve into the solution (known as ionization tendency).
- Zinc (Zn): “I really want to dissolve!” It wants to become an ion (Zn²⁺) even if it has to throw away its electrons (e⁻).
- Copper (Cu): Not as eager to dissolve. In fact, the copper ions (Cu²⁺) in the solution would rather receive electrons (e⁻) and turn back into solid copper (Cu).
This “Zinc wanting to get rid of electrons” and “Copper ions wanting electrons” is the whole secret behind the battery!
Step-by-Step Electricity Flow
- 【Negative Terminal: Zinc Side】 Zinc dissolves and kicks out electrons!
The zinc plate (Zn) releases two electrons (e⁻) and transforms into a “zinc ion (Zn²⁺),” which dissolves into the zinc sulfate solution.
Zn → Zn²⁺ + 2e⁻ (Releases electrons)
As a result, the zinc plate gradually dissolves and becomes ragged. The released electrons (e⁻) travel through the wire toward the copper plate. This movement is the electric current!
- 【Positive Terminal: Copper Side】 Copper ions catch the electrons!
The electrons (e⁻) arrive at the copper plate via the wire. The blue “copper ions (Cu²⁺)” waiting in the solution rush to the plate and grab those two electrons.
Cu²⁺ + 2e⁻ → Cu (Receives electrons)
The copper ions that received the electrons turn back into regular “copper (Cu)” atoms. As a result, the copper plate gets thicker as new copper attaches to it, and the blue color of the solution fades.
What is the Cellophane for?
The cellophane is a “partition” that prevents the two liquids from mixing immediately. But it’s not just a wall. On the zinc side, positive ions (Zn²⁺) increase, while on the copper side, positive ions (Cu²⁺) decrease. To prevent an electrical imbalance, the tiny holes in the cellophane allow “negative ions (like SO₄²⁻)” to move back and forth, playing a vital role in maintaining the cell’s electrical balance.
For more details on the standard Daniel Cell, please check out this article as well!
Inquiries and Requests
Bringing the wonder and fun of science closer to you! I share easy-to-follow tips and fun experiments you can try at home. Feel free to explore more!
Learn more about Ken Kuwako here
For work requests (writing, lectures, workshops, TV supervision, etc.), click here – Follow me on X (Twitter) for updates!
![]() Watch experiment videos on the Science Material Channel!
Watch experiment videos on the Science Material Channel!


