Unveiling the Mystery of the “Zap!” — The Shocking Secrets of the 150,000-Volt Van de Graaff Generator!
I’m Ken Kuwako, a Science Trainer, and every day is an experiment.
When winter rolls around, we all know that sudden “Zap!” when you touch a doorknob, or the crackling sound when you pull off a sweater. These are all the work of “static electricity,” where materials build up an electrical charge. Today, I’m going to introduce a slightly suspicious—but ultimately fascinating and incredibly fun—piece of scientific equipment that lets you deliberately generate and (almost) control this static electricity.
Mastering the “Zap”? Meet the Mysterious Sphere: The Van de Graaff Generator
The device is called the “Van de Graaff generator.” Have you ever been in a science lab or museum and seen someone touch a silver sphere and their hair suddenly stand straight up, going “poof!”? That’s the one. This slightly hard-to-remember name actually comes from the inventor, Dr.Robert Jemison Van de Graaff.
What’s fascinating is that this wasn’t originally created as a toy for science class. Dr. Van de Graaff developed this high-voltage generator as part of a “particle accelerator,” a machine that smashes particles into atomic nuclei at high speeds. In other words, it was originally cutting-edge research equipment designed to explore the atomic world. (If you’re curious, look up the “Van de Graaff Accelerator” to appreciate its original, grand scale!)
(By the way, I’ve summarized the mechanism of “Elekiter,” a static electricity generator from the Edo period in Japan, here.)
The 150,000-Volt Club!
The one I personally own is a Van de Graaff generator made by Narika. Believe it or not, it can generate a whopping 150,000 volts! (I tried to buy it secretly without telling my family, but given its size… they found out immediately.)
Hearing “150,000 volts” might make you think, “Danger! Electrocution!” However, while the voltage (volts) of static electricity is high, the amount of current (amps) flowing is extremely small, so even though you’ll feel a sharp tingle, there’s no major danger. (Of course, caution is necessary for those with heart conditions or pacemakers!) Normally, static electricity experiments are difficult to pull off unless it’s a dry winter day, but because this machine is so powerful, you can do sparking, crackling experiments even on humid days.
The Van de Graaff generator allows you to perform experiments like making hair stand up or building up and discharging electricity. For example, when you touch the generator, your body accumulates an electric charge, which then discharges from your fingertips, causing a sharp “snap!” By cleverly using this mechanism, you can do all sorts of fun static electricity experiments!
Surprisingly Simple? What’s Inside the Van de Graaff Generator
The Van de Graaff generator might look like a device straight out of a mad scientist’s lab, but what’s really inside? You can actually pop open the sphere. Come on, let’s take a peek inside…

Surprised? That’s right—all it contains is something like a conveyor belt. Checking the specifications for my Narika-made Van de Graaff generator on their website shows this…
Static electricity generated by the rubber belt rollers is guided to the dome (collecting sphere), where it is stored to generate high-voltage static electricity.
● Generating Voltage: Max. approx. 150,000V ● Discharge Distance (approx.): Max. 110mm (at 40% humidity), 60mm or more during the rainy season (actual measurement) ● Collecting Sphere: approx. φ215mm ● Discharging Sphere: approx. φ115mm ● Power Supply: AC100V 50/60Hz ● Dimensions: approx. 270×210×620mm (collecting sphere), approx. 150×130×490mm (discharging sphere) ● Weight: approx. 5.6kg
The Heart of the Van de Graaff: The Amazing Mechanism for “Carrying” Electricity
In short, the principle of the Van de Graaff generator is: “Create electricity through friction, carry it with a belt, and store it in a sphere.” When we popped open the sphere, we saw a rubber belt and two rollers, one at the top and one at the bottom. In fact, the biggest secret of the Van de Graaff is the simple act of changing the material of these two rollers. Let’s trace the belt’s movement from the moment the switch is flipped until the charge builds up.
① [Bottom Section] Rubbing to “Create” Electricity (Generating Negative Charge)
First, when the conveyor belt starts moving, the rubber belt rubs strongly against the lower roller A (made of acrylic resin). Materials have a sequence of properties, known as the “triboelectric series,” which determines whether they tend to become “positive” or “negative” when rubbed together.
In the case of rubber and acrylic resin, acrylic resin tends to become positive, and rubber tends to become negative. This means that negative electricity (electrons) jump from the acrylic resin to the rubber belt. As a result, the rubber belt becomes charged with a negative (-) charge.
② [Ascent] Carrying and “Storing” the Charge (To the Sphere)
The rubber belt, now heavily laden with a negative (-) charge, rapidly ascends like a conveyor belt. It then arrives at a metal “collector comb” inside the upper sphere. The negative charge (electrons) carried by the belt discharges onto the metal collector comb with a sharp crackle. Since electrons can move freely inside metal, the charge instantly spreads across and accumulates throughout the entire sphere.
③ [Top Section] Rubbing to “Reset” the Charge (Generating Positive Charge)
The rubber belt, having delivered its charge and now “empty,” changes direction and descends. At this point, it rubs against the upper roller B (made of polyvinyl chloride resin). Here’s where the “triboelectric series” comes into play again! The new partner is polyvinyl chloride resin, which has a property of being “even more likely to become negative” than rubber. This time, the opposite happens: negative electricity (electrons) is stripped away from the rubber belt and transferred to the polyvinyl chloride resin. The rubber belt, having lost electrons, becomes relatively charged with positive (+) electricity.
④ [Descent] Carrying the Opposite Charge (To the Base)
The rubber belt, now charged with positive (+) electricity, descends to the lower section. When it reaches the second collector comb at the bottom, it transfers the positive charge there. This positive electricity is then accumulated in the “support base” (the foundation) of the Van de Graaff generator.
⑤ [Repetition] And On to High Voltage…
The belt continues to touch the lower roller A (acrylic resin) and returns to step ①: “taking on a negative (-) charge.” This cycle—“① carry negative charge → ② store it in the sphere → ③ carry positive charge → ④ store it in the base”—is repeated at high speed by the motor.
As a result, a negative (-) charge rapidly builds up in the upper sphere, and a positive (+) charge builds up in the lower support base. By accumulating massive amounts of negative and positive charges in separate locations, a strong force (“desire for electricity,” or voltage) is created between the two, resulting in the colossal 150,000 volts.
Static electricity is not just about rubbing things together; by combining the choice of materials and the transport mechanism, you can generate such powerful energy. That truly is scientific ingenuity!
A common misconception is that electrons are flowing in from the power outlet. Surprisingly, the mechanism is simple. The power outlet’s electricity is only used to run the motor; the electricity itself is created by “friction.”
You can check out a more detailed explanation of the mechanism here.
Experiment Time! Is the Sphere Charged Positive or Negative?
Of course, you never really know until you try it! So, I also investigated which charge—positive or negative—accumulates on the top sphere of this static electricity generator.
https://youtu.be/1u3j8XRYNrg
We’ll use a “balloon.” 
If you rub a balloon with tissue paper or similar material, the balloon becomes negatively charged.
Materials contain both “positive” (+) and “negative” (-) charges, and when two different materials are rubbed together, electrons (negative charge) tend to move. Between a balloon and tissue paper, electrons move from the tissue paper to the balloon, so the balloon becomes negatively charged.
Now, let’s bring this negatively charged balloon close to the powered-on Van de Graaff generator. I’m using the Narika model. After turning it on, I bring the balloon near…

When I let go, it was repelled.
The balloon was clearly repelled! Recall the basic rule of science: “Like charges (- and -, or + and +) repel each other, while opposite charges (+ and -) attract.” Since the negatively charged balloon was repelled, we know that the sphere of this Van de Graaff generator must also be accumulating a negative charge!
[Advanced] Does Electricity “Run Away”? The Mystery of Electrostatic Induction
Here’s where things get interesting: after turning the Van de Graaff switch OFF, I touch the sphere with my hand to let the charge escape (discharge it). The sphere should now be in a “neutral” state—neither positive nor negative, or “empty” of charge. So, what happens when we bring the negative balloon close to this “empty” sphere?
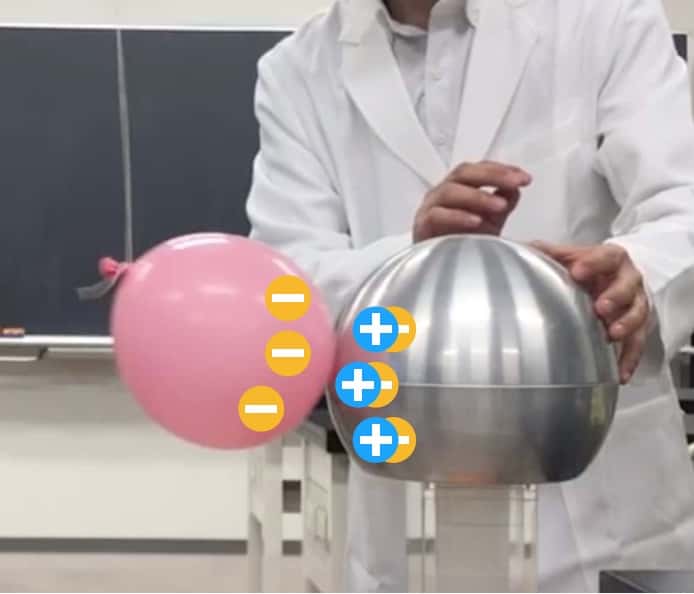
Amazingly, the balloon suddenly sticks to it! Isn’t that weird? If the sphere is “empty,” why would it attract the negative balloon (thereby exhibiting a positive property)? This is one of the most fascinating phenomena of static electricity, called “electrostatic induction.”
The sphere is made of “metal.” Inside metal, there are many negative charges (free electrons) that can move around freely. When the negative balloon approaches… the free electrons inside the metal shout, “Whoa! A negative charge is coming!” and flee to the side furthest away from the balloon (which, in this photo, is the side my hand is touching).
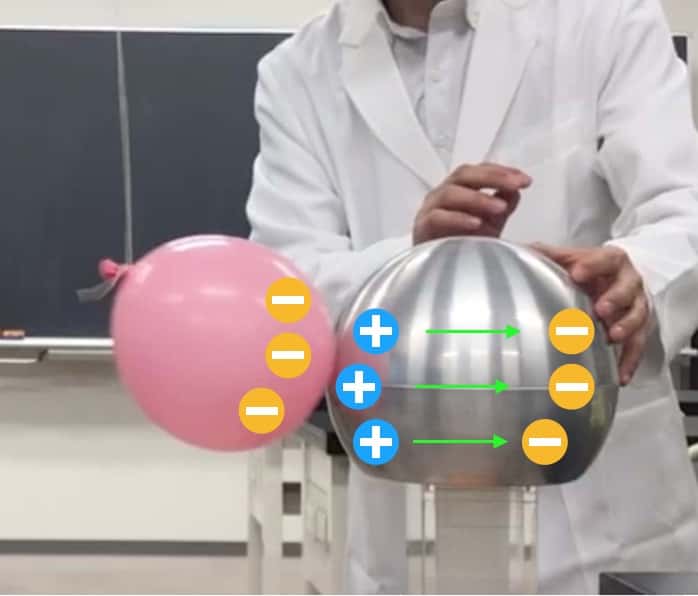
Negative charge moves to the opposite side of the sphere (electrons may be escaping through my body)
As a result, the side closer to the balloon becomes “positive” because the electrons have left that area. That’s why it attracts the negative balloon. This is the same principle that explains why hair, rubbed with a plastic ruler, sticks to a wall that shouldn’t be charged.
Static Electricity is a Treasure Trove of “Whys?”
It’s a small change, but the balloon’s movement switches from “repulsion” when the power is ON to “attraction” when the power is OFF. Pondering why these everyday phenomena occur is the true depth of static electricity experiments and the essence of science itself.
The next time you feel a “Zap!” in winter, it’s proof that electrons (negative charges) either jumped from you to something else or from something else to you. Reflecting on it and thinking, “Ah, an electron just moved,” can make the experience a little more fun.
Check Out These Cool Experiments You Can Do With a Static Electricity Machine (Van de Graaff)!
I’ve also shared some fun experiments using the Van de Graaff generator. These include experiments conducted on a TV program with celebrities like Suzu Hirose, Ryohei Suzuki, Yasuko, and Osada & Matsuo from Chocolate Planet. Find out more here.

※ Please ensure that all experiments using a static electricity generator (Van de Graaff) are conducted under the supervision of a specialist. Please proceed with caution. For requests regarding static electricity experiments (science classes, TV supervision/appearances, etc.), please contact me here.
[Feature Article] You Can’t Stop Once You Start! Static Electricity Experiments
Contact and Requests
Bring the wonder and fun of science closer to you! I’ve put together easy-to-understand guides on fun science experiments you can do at home, along with tips and tricks. Feel free to search around! ・The content of this science blog is now available as a book. Details here. ・About the operator, Ken Kuwako, click here. ・For all requests (writing, lectures, science classes, TV supervision/appearances, etc.), click here. ・Article updates are posted on X!
![]() Experiment videos are available on the Science Topics Channel!
Experiment videos are available on the Science Topics Channel!




