The Science of the ‘Pop!’: Making Hydrogen Gas at Home
I’m Ken Kuwako, your Science Trainer. Every day is an experiment.
Have you ever heard of a “clean gas that turns into water when burned”—a substance that sounds like magic? Well, that’s hydrogen! This time, let’s dive into an exciting chemistry experiment to generate this potential fuel of the future using simple, everyday materials!
Challenge Accepted: Generating Hydrogen!
For this experiment, we’ll be generating hydrogen using zinc and hydrochloric acid. We conducted an oxygen generation experiment previously, so it’ll be fascinating to compare the differences in their properties.
First Things First: Preparation!
To generate hydrogen gas, you’ll need the following materials and equipment.
Materials
•Zinc (mossy or granular) 1g •10% Hydrochloric Acid 7mL

Equipment
•Test tubes (2) •Matches •Glowing splint (a smoldering stick/splinter) •Wet cloth (essential for safety!)
Procedure for Collecting Hydrogen
① Time to Generate Hydrogen!

Pour the diluted hydrochloric acid into a test tube, and then add the mossy zinc. What happens next? Tiny bubbles vigorously fizz and pop! This gas is our star of the show: hydrogen. In the world of chemistry, this reaction is represented like this:
Zn (亜鉛) + 2HCl (塩酸) ⟶ ZnCl₂ (塩化亜鉛) + H₂ (水素)
The zinc dissolves into the hydrochloric acid, transforming into a substance called zinc chloride while simultaneously releasing hydrogen gas.
We’ll collect the generated hydrogen using the water displacement method. If the bubbling slows down, especially in the winter, slightly warming the test tube with hot water will help speed up the reaction!

② Bring the Flame Closer!
Now, take a lit match and bring it close to the test tube containing the collected hydrogen. (Note: Use the second tube of collected gas, as the first one might contain air mixed in!) Time for a quiz! What do you think will happen when the flame gets close?
The answer is… it burns with a small “pop!” sound!
This “pop” is proof that the hydrogen violently reacted (combusted) with the oxygen in the air, changing into water (H₂O). The sound is essentially a “miniature explosion” caused by the reaction’s energy instantly expanding the surrounding air. If you try this yourself, you’ll discover that the sound changes—sometimes a “whoosh!” or a “squeak!”—depending on the conditions, which is a fun observation!
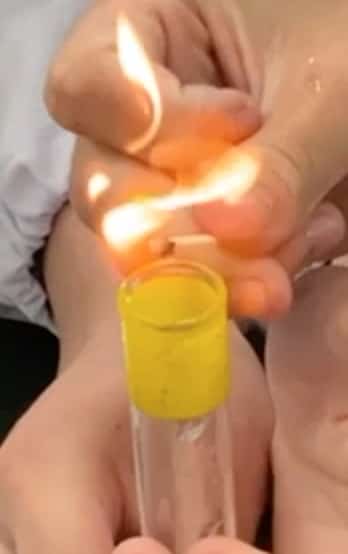
③ One More Experiment!
If you have another test tube of collected hydrogen, try adding limewater and shaking it. In the oxygen experiment, there was no change in the limewater, but what about this time? …Most likely, there will be no change. Limewater turns cloudy only in the presence of carbon dioxide, which shows us that hydrogen and oxygen have different properties than CO₂.
Diving Deeper into Science: The Mystery of the Zinc’s Shape
We used “mossy zinc” for this experiment. Interestingly, the shape of the zinc dramatically affects how easily hydrogen is generated. Why would this be, if it’s the same chemical substance?
The hint is “Surface Area”!
Imagine a sugar cube versus powdered sugar—which one dissolves faster in water? Powdered sugar, of course! That’s because powdered sugar has a much larger area touching the water, or a larger surface area.
It’s the same principle here: the complex shape of mossy zinc has a larger surface area exposed to the hydrochloric acid compared to smooth, granular zinc. This larger contact area allows more reactions to occur, generating hydrogen much more vigorously. Pondering these “whys” is the real thrill of science! After the experiment, the zinc loses its metallic luster and turns dark. This happens because the zinc dissolves or another substance forms on the surface. If you ever plan to reuse it, you’ll need to sand it down to expose the shiny metal underneath.

Left: Before experiment Right: After experiment
Conclusion
Our students quickly got the hang of handling the equipment and skillfully completed the experiment in under an hour. Their progress is truly inspiring! When you look at everyday phenomena through the lens of chemistry, you’ll uncover a wealth of new discoveries. Go ahead and search for your next “Why?”
Contact & Collaboration
We aim to make the wonders and fun of science more accessible! We offer clear instructions for fun science experiments you can do at home, along with tips and tricks. Feel free to search around!
・The content from this science blog is now available as a book. Find out more here.
・Learn more about the administrator, Ken Kuwako, here.
・For all inquiries (writing, lectures, science classes, TV supervision/appearances, etc.), please contact us here.
・Article updates are posted on X!
![]() We stream experiment videos on the Science Neta Channel!
We stream experiment videos on the Science Neta Channel!
3月のイチオシ実験!
- 押し花を作ろう!:梅や桜の花の押し花を作ってみましょう。特別なケースに入れると、長く保存できて、しおりにもなります。
テレビ番組・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
- 2月27日放送予定「チコちゃんに叱られる」(NHK)の科学監修しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師・ショー・その他お知らせ
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) 秘密兵器「帯電ガン」が炸裂!ビリビリ!ドキドキ!静電気サイエンスショー@千葉市科学フェスタ(午後予定)
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。



