How to Dilute Hydrochloric Acid, Sulfuric Acid, Ammonia, and Copper Chloride: Master the Basics of Dilution for Safe Lab Prep!
I’m Ken Kuwako, a science trainer. For me, every day is an experiment.

【Read this article in English】
When handling chemicals in the science lab, a solid understanding of dilution is essential for conducting experiments “safely, accurately, and efficiently.” In this guide, I’ve put together key dilution methods, safety protocols, and practical concentration levels for common experiments, specifically for fellow teachers. This is based on research I originally did for my own preparations. Knowing you’ve “done your homework” is a great confidence booster, and thorough preparation is the backbone of a quality lesson. This article will help you get a firm grasp on how to think about diluting the chemicals you use most often.
For example, science handbooks like Hamajima Shoten’s Science Handbook (page 243) provide summaries of dilution methods. From these, we know that a 5% concentration of sodium hydroxide works for the electrolysis of water, and a 5% concentration of hydrochloric acid is fine for its own electrolysis. This page, however, offers a more detailed, practical guide on solution preparation for teachers in the field. For the specific calculations, like converting from mass percent concentration to molarity, please see my other article here:
How to Dilute Hydrochloric Acid
For the hydrochloric acid commonly used in middle school science experiments, it’s often best to dilute a batch from concentrated HCl all at once. To make a 10% solution, you can add 3.2 parts water to 1 part concentrated HCl by volume. For instance, to convert an entire 500 cm³ bottle of concentrated HCl to a 10% solution, you would dilute it with 1600 cm³ of water. It’s convenient to prepare and store this in a 3-liter polyethylene container (Amazon).

|
|
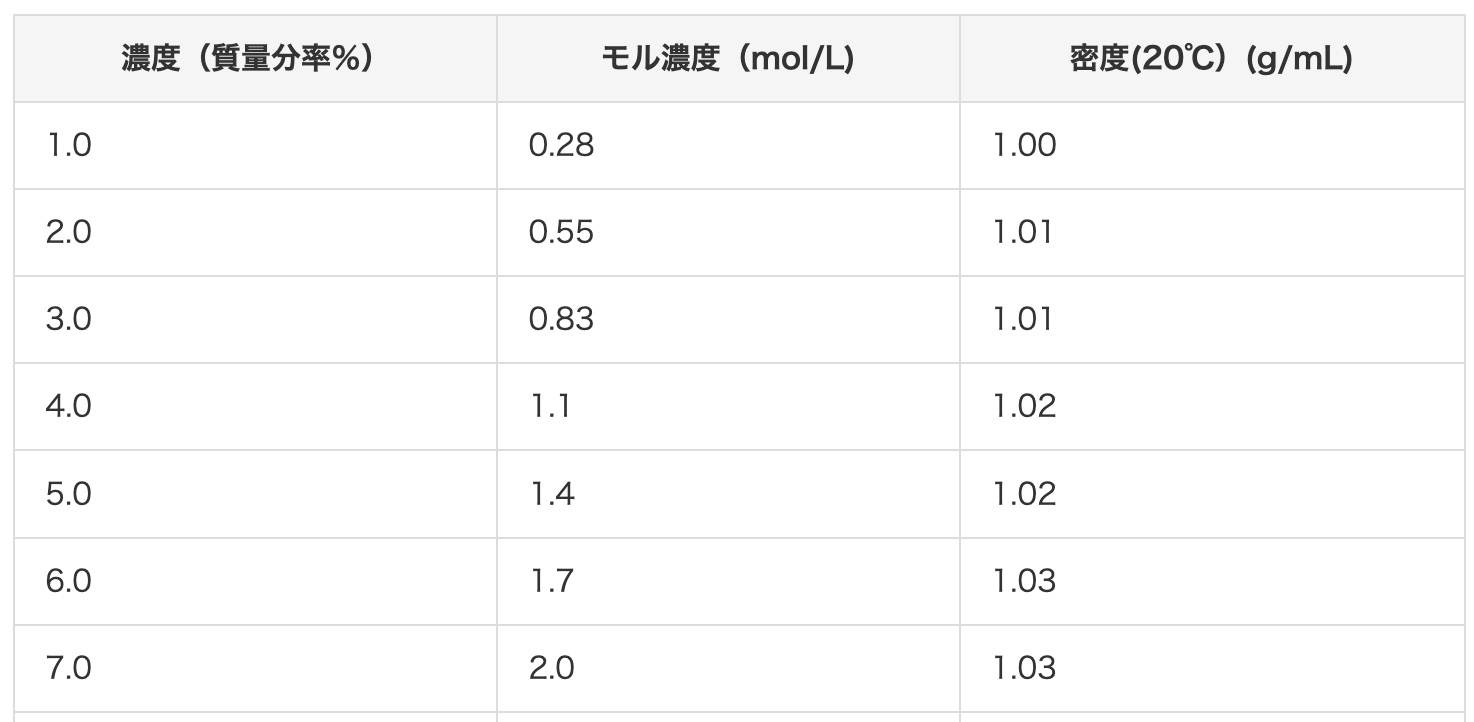
You can also refer to the conversion chart for hydrochloric acid concentrations on this site.
For a demonstration of the dilution method, please watch this video.
If you’re unsure, let’s double-check with the formula.
For example, concentrated hydrochloric acid is 12 mol/L and has a density of 1.18 g/mL. Therefore, the mass of 1L (1000 mL) of the solution is:
1.18 × 1000 = 1180 g
With a molecular weight of 36.5 for HCl, the mass of the solute is:
12 × 36.5 = 438 g
Therefore, the mass percent concentration of the concentrated HCl is:
438 / 1180 = 37.1%
To dilute this to 10% by adding water, let x[g] be the mass of water to add:
438 / (1180 + x) = 10 / 100
x = 3200 g
This means that for every 1L of hydrochloric acid, you need to add 3.2L of water to get a 10% solution. If you have 500 mL of HCl, you’d use half that amount, which is 1.6L of water.
Safety Note
When diluting hydrochloric acid, always add the acid to the water. Adding water to acid (the wrong way!) can cause the solution to heat up and splash dangerous chemicals. I have compiled some useful sites regarding the dilution methods for hydrochloric and sulfuric acid. Incidentally, the difference in concentration between concentrated HCl and concentrated H₂SO₄ is due to their different solubilities.

A 9% concentration is approximately 2.6 N (2.6 mol/L).
A 4% concentration is approximately 1 N (1 mol/L).
Reference: http://www.keins.city.kawasaki.jp/9/ke9004/benrihp060821/2020.benrichou.ensan.pdf
Concentrated hydrochloric acid is 12 mol/L. Therefore, to make a 1 mol/L solution, you need to dilute it 12 times. This means the total volume is 12 parts, with 1 part being acid. So, you should mix at a ratio of 1 part acid to 11 parts water.
※Important: When diluting, adding water to an acid or base can generate heat from dissolution, causing the water to boil and splash, which is dangerous. Always add the acid or base to the water when diluting.
[For Hydrochloric Acid]
・Concentrated HCl is 12 mol/L. To make a 1 mol/L solution, dilute it 12-fold. This means a ratio of 1 part HCl to 11 parts water.
(Example) Add 10 mL of HCl to 110 mL of water.
※Important: Always add acid to water, as the reverse can cause dangerous splashing due to heat generation.
[For Ammonia Solution]
・Concentrated ammonia is 14 mol/L. To make a 1 mol/L solution, mix at a ratio of 1 part concentrated ammonia to 13 parts water.
※ For liquid solutes, molarity is used. For solid solutes, you can prepare a solution of a desired concentration if you know the molecular weight.Reference: http://www.edu.pref.kagoshima.jp/curriculum/rika/shou/syougakkou2/gihouhtm/01page/page03.htm
How to Dilute Sodium Hydroxide
Quick Reference Chart for Sodium Hydroxide Solution Concentrations
[For Sodium Hydroxide]
・One mole of sodium hydroxide (NaOH) is 40g. Therefore, dissolving 40g of NaOH in water to make a total volume of 1L creates a 1 mol/L solution.
To make 100 mL of a 1 mol/L (4%) NaOH solution, dissolve 4g of NaOH in water and bring the total volume to 100 mL.
※ Dissolving sodium hydroxide in water is an exothermic reaction. It’s easier to dissolve it in a small amount of water first, letting the temperature rise, and then add the rest of the water.Reference: http://www.edu.pref.kagoshima.jp/curriculum/rika/shou/syougakkou2/gihouhtm/01page/page03.htm
◆ For preparing large volumes (approx. 500 mL or more)
Use a graduated cylinder to measure the required amount of distilled water (e.g., 500 mL for a 500 mL solution). Pour half of the water into a large beaker A, leaving the rest in the cylinder.
Using a balance, weigh out the sodium hydroxide in a separate beaker B. Immediately add the NaOH to the distilled water in beaker A and stir well. Use the remaining water from the graduated cylinder to rinse any NaOH stuck to the bottom of beaker B and add it to beaker A.
If needed, transfer the solution to a reagent bottle for storage. Be sure to label it with the chemical name, concentration, preparation date, and your name.
◆ For preparing small volumes (less than 500 mL)
Use a graduated cylinder to measure the required amount of distilled water (e.g., 100 mL for a 100 mL solution).
Using a balance, weigh the sodium hydroxide into a beaker. Immediately add the measured distilled water and stir well.
If needed, transfer to a reagent bottle and label it with the chemical name, concentration, date, and creator.
Concentration for Electrolysis of Copper(II) Chloride
For the electrolysis of copper(II) chloride, I prepared the solution by taking 13g of CuCl₂ and adding distilled water to make 300g of solution. This results in an approximately 3% copper(II) chloride solution. You can find more details here. Although it’s a dilute solution, it works perfectly well for the experiment.
Concentrations for a Daniell Cell
#ZincSulfate solution: 5% – 9.8g in 100g of water. #CopperSulfate solution: Approx. 17% – 35.7g in 100g of water.
I have summarized the details here.
Other Helpful Resources
*Experiments to test for acidity/alkalinity: For these experiments, you just need enough concentration to change the color of litmus paper, so about 0.3 to 1 mol/L is usually sufficient. For a 0.3 mol/L solution, a ratio of concentrated HCl to water of 1:39 works. *Experiments to generate hydrogen with metals: To dissolve metal, you need a higher concentration. Dilute to about 3 mol/L. A ratio of concentrated HCl to water of 1:3 is suitable.
Reference: https://www.ice.or.jp/nc/?action=common_download_main&upload_id=1169
◆ In Conclusion
Worries like, “Was that too concentrated?” or “Should I have diluted it more?” can be eliminated with knowledge and preparation. By mastering proper dilution techniques, you not only increase safety in your experiments but also demonstrate good scientific practice to your students. Let’s continue to share the fun and depth of science, always remembering to handle chemicals with care!
Inquiries & Requests
Bringing the wonder of science closer to home! I create easy-to-understand guides for fun science experiments you can do at home. Feel free to search for topics that interest you!
・About the author, Ken Kuwako, click here.
・For requests (writing, lectures, workshops, TV consulting, appearances, etc.), click here.
・Follow me on X (Twitter) for article updates!
![]() My Science Topics Channel features experiment videos!
My Science Topics Channel features experiment videos!
2月のイチオシ実験!梱包材で遊ぼう!
- 静電気の時期になってきました。子供と一緒に梱包材で盛り上がろう!→ やめられなくなる!静電気実験20
 体中に梱包材をはりつけてみよう!
体中に梱包材をはりつけてみよう!
テレビ番組等・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師等・ショー・その他お知らせ
- 2/20(金)「生徒の進学希望実現支援事業」研究授業@福井県立若狭高等学校 講師
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) サイエンスショー予定
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。

![[Product price may have changed since this link was created.] [Product price may have changed since this link was created.]](https://hbb.afl.rakuten.co.jp/hgb/2c6a2576.25faaa1b.2c6a2577.0abda625/?me_id=1240371&item_id=10000489&pc=https%3A%2F%2Fthumbnail.image.rakuten.co.jp%2F%400_mall%2Fnagamineshouten%2Fcabinet%2Fporikan%2F3-1.jpg%3F_ex%3D80x80&s=80x80&t=picttext)

