Hydrochloric Acid, Sulfuric Acid, Ammonia, and Copper Chloride Dilution Methods (Master the Basics of Dilution! ~Correct Chemical Handling and Lesson Preparation~)
I’m Ken Kuwako, a science trainer. Every day is an experiment.

When handling chemicals in the science lab, a basic knowledge of dilution is essential for conducting experiments “safely, accurately, and efficiently.” This time, I’ve put together a guide for teachers on the dilution methods, safety aspects, and concentration guidelines for common experiments that you should know. This is based on my own research, and knowing that you’ve prepared properly provides a sense of security. Preparation without mistakes supports the quality of your lessons. By reading this article, you can get a solid grasp of how to think about diluting frequently used chemicals.
For example, on page 243 of Hamajima Shoten’s Science Handbook, you’ll find a summary of dilution methods and preparation instructions. It shows that a 5% concentration is fine for the electrolysis of sodium hydroxide, and a 5% concentration of hydrochloric acid is also sufficient for its electrolysis. On this page, I’ve compiled a more detailed, practical guide to preparing these solutions for teachers in the field. For calculation methods, such as converting from mass percentage concentration to molar concentration, please refer to a separate article. You can find it here:
About Hydrochloric Acid Dilution
Hydrochloric acid is often used in middle school science experiments, and it’s recommended to dilute concentrated hydrochloric acid all at once for use. To make a 10% solution, you can add 3.2 parts water to 1 part concentrated hydrochloric acid by volume. For example, to make the entire 500 cm³ of concentrated hydrochloric acid a 10% solution, you’d dilute it with 1600 cm³ of water. It’s convenient to dilute and store it in a 3-liter polyethylene container (Amazon).

|
|
You can also refer to the hydrochloric acid concentration conversion chart on this site.
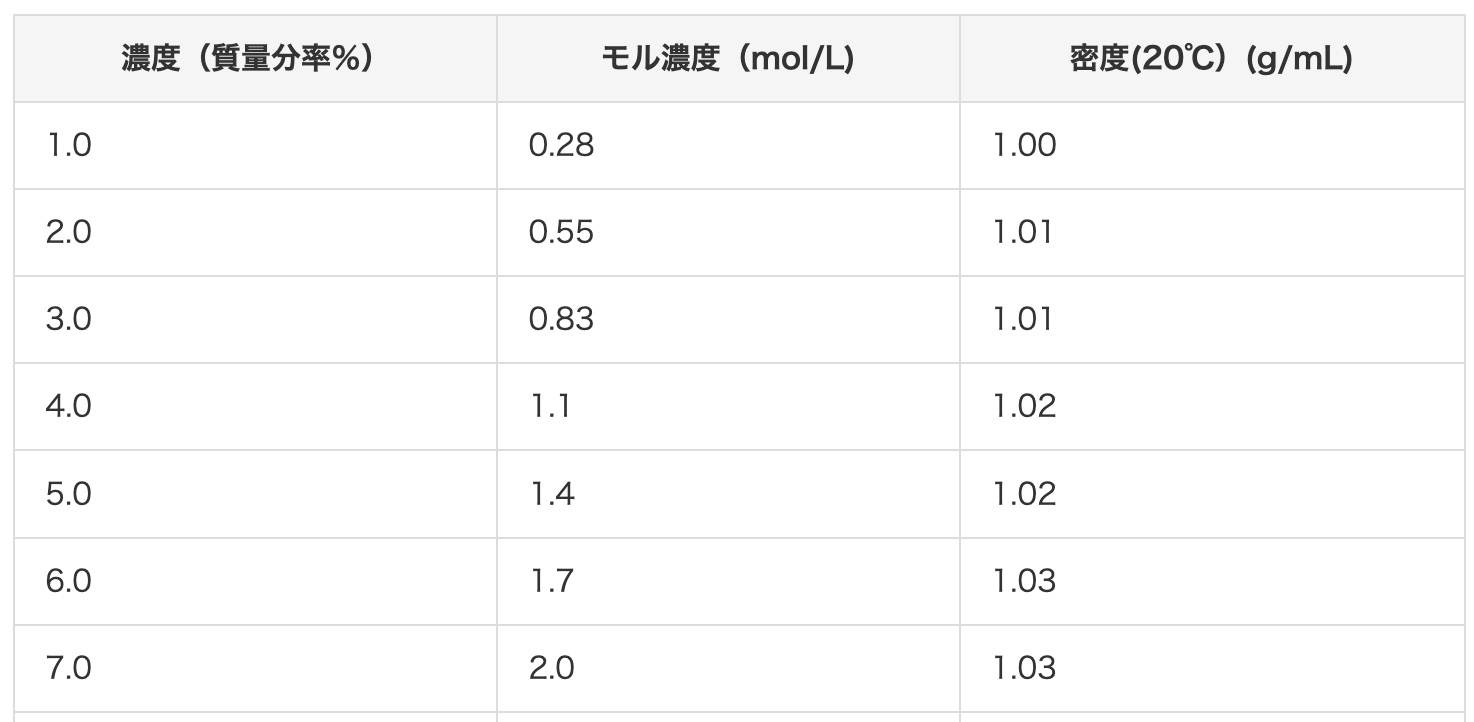
For dilution methods, please see here:
If you’re worried, let’s also check the calculations.
For example, concentrated hydrochloric acid is 12 mol/L, and its density is 1.18 g/mL. So, the mass of a 1L (1000 mL) solution of concentrated hydrochloric acid is:
1.18 × 1000 = 1180 g
Also, the mass of the solute, with a molecular weight of 36.5, is:
12 × 36.5 = 438 g
Therefore, the mass percentage concentration of concentrated hydrochloric acid is:
438 / 1180 = 37.1%
To dilute this concentration to 10%, if the amount of water to be added is x [g], then:
438 / (1180 + x) = 10 / 100
x = 3200 g
This means that you need to add 3.2 L of water to 1 L of hydrochloric acid to make a 10% concentration. If you have 500 mL of hydrochloric acid, you would add half of that, which is 1.6 L.
Important Note:
When diluting hydrochloric acid, make sure you add the acid to the water. If you add water to an acid or alkali (×), the heat of dissolution can cause the added water to splash out, which is dangerous. I’ve compiled some helpful websites on how to dilute hydrochloric acid and sulfuric acid. By the way, the difference in concentration between concentrated hydrochloric acid and concentrated sulfuric acid is due to the difference in solubility.

For a 9% concentration, the normality N is approximately 2.6 (2.6 mol/L)
For a 4% concentration, the normality N is approximately 1 (1 mol/L)
Reference: http://www.keins.city.kawasaki.jp/9/ke9004/benrihp060821/2020.benrichou.ensan.pdf
The concentration of concentrated hydrochloric acid is 12 mol/L. Therefore, to make a 1 mol/L concentration, you need to dilute it 12 times. The total volume is 12 parts, with 1 part being hydrochloric acid, so you should mix it in a ratio of hydrochloric acid 1 : water 11.
※Caution: When diluting, adding water to an acid or alkali (×) can cause dangerous splashing due to the heat of dissolution. Always add the acid or alkali to the water.
【For Hydrochloric Acid】
– The concentration of concentrated hydrochloric acid is 12 mol/L. Therefore, to make a 1 mol/L concentration, you need to dilute it 12 times. The total volume is 12 parts, with 1 part being hydrochloric acid, so you should mix it in a ratio of hydrochloric acid 1 : water 11.
(Example) Add 10 mL of hydrochloric acid to 110 mL of water.
※Caution: When diluting, adding water to an acid or alkali (×) can cause dangerous splashing due to the heat of dissolution. Always add the acid or alkali to the water.
【For Ammonia Water】The concentration of concentrated ammonia water is 14 mol/L. Therefore, to make a 1 mol/L ammonia water solution, you should mix concentrated ammonia water 1 : water 13.
※If the solute is a liquid, you can make a solution of the desired concentration using molarity. If it’s a solid, you can do so if you know the molecular weight.Reference: http://www.edu.pref.kagoshima.jp/curriculum/rika/shou/syougakkou2/gihouhtm/01page/page03.htm
About Sodium Hydroxide Dilution
Sodium Hydroxide Solution Concentration Quick Reference Chart
【For Sodium Hydroxide】
1 mole of sodium hydroxide is 40 g. Therefore, when 40 g of sodium hydroxide is dissolved in water to make 1 L, the concentration is 1 mol/L.
To make 100 mL of a 1 mol/L (4%) sodium hydroxide solution, dissolve 4 g of sodium hydroxide in water to make 100 mL.
※When dissolving sodium hydroxide in water, it causes an exothermic reaction. Therefore, it is easier to dissolve it in a small amount of water to raise the temperature first, and then dissolve the rest.Reference: http://www.edu.pref.kagoshima.jp/curriculum/rika/shou/syougakkou2/gihouhtm/01page/page03.htm
◆When making a large volume solution (approx. 500 mL or more)
Use a graduated cylinder that can measure the volume you need to make (for example, a 500 mL or 1000 mL cylinder to make 500 mL). Measure the distilled water, transfer half of it to a large beaker A, and leave the rest in the graduated cylinder.
Use a balance to measure the sodium hydroxide into a separate beaker B, and immediately add it to the distilled water in beaker A from step 1, stirring well. Transfer any sodium hydroxide sticking to the bottom of beaker B from step 2 to beaker A from step 1 after dissolving it with the remaining distilled water from the graduated cylinder.
If necessary, transfer it to a reagent bottle for storage. Be sure to label it with the reagent name, concentration, date of creation, and creator’s name.
◆When making a small volume solution (approx. less than 500 mL)
Use a graduated cylinder (for example, a 100 mL or 200 mL cylinder to make 100 mL) to measure the distilled water.
Use a balance to measure the sodium hydroxide into a beaker, and immediately add the distilled water you measured in step 1 and stir well.
If necessary, transfer it to a reagent bottle for storage. Be sure to label it with the reagent name, concentration, date of creation, and creator’s name.
About Concentration Adjustment for Copper Chloride Electrolysis
For adjusting the copper chloride solution used in electrolysis, I used 13 g of copper chloride and added distilled water to make a 300 g solution. This is approximately a 3% copper chloride solution. You can find more details here. The experiment works well even with this weak solution.
About Concentration Adjustment for the Daniell Cell
#ZincSulfate solution: 5% (9.8 g in 100 g of water) #CopperSulfate solution: approximately 17% (35.7 g in 100 g of water)
I have summarized the details here.
Other Useful Reference Sites
*Experiments to test for acidity/alkalinity: For these experiments, it’s enough for the litmus paper to change color, so a concentration of about 0.3 to 1 mol/L is sufficient. For 0.3 mol/L, the ratio is concentrated hydrochloric acid : water = 1:39. *Experiments to generate hydrogen by adding metal: For experiments that dissolve metals, a certain concentration is required. Dilute to about 3 mol/L. The ratio is concentrated hydrochloric acid : water = 1:3
Reference: https://www.ice.or.jp/nc/?action=common_download_main&upload_id=1169
◆Closing Remarks
Anxiety like “Was it a bit too concentrated?” or “Should I have diluted it more?” can be eliminated with prior knowledge and preparation. By properly mastering dilution methods, you not only improve safety during experiments but also convey the correct scientific attitude to your students. While always taking great care in handling chemicals, let’s continue to share the fun and depth of science!
About Inquiries and Requests
Make the wonders and fun of science more accessible! I’ve summarized fun at-home science experiments and their tips in an easy-to-understand way. Please search around!
・For more about the operator, Ken Kuwako, click here
・For various requests (writing, lectures, science classes, TV supervision, appearances, etc.), click here
・Article updates are delivered on X!
![]() The Science Neta Channel is delivering experiment videos!
The Science Neta Channel is delivering experiment videos!

![[商品価格に関しまcしては、リンクが作成された時点と現時点で情報が変更されている場合がございます。] [商品価格に関しましては、リンクが作成された時点と現時点で情報が変更されている場合がございます。]](https://hbb.afl.rakuten.co.jp/hgb/2c6a2576.25faaa1b.2c6a2577.0abda625/?me_id=1240371&item_id=10000489&pc=https%3A%2F%2Fthumbnail.image.rakuten.co.jp%2F%400_mall%2Fnagamineshouten%2Fcabinet%2Fporikan%2F3-1.jpg%3F_ex%3D80x80&s=80x80&t=picttext)

