Just Mix It and Reach 80°C!? Discover the Heat of Rust with a DIY Hand Warmer! Learn the Science of Exothermic Reactions by Making Your Own
I’m Ken Kuwako, a Science Trainer. Every Day is an Experiment.
On a cold winter day, the gentle warmth of a heat pack tucked into your pocket feels like a gift from the heavens, doesn’t it? But have you ever stopped to imagine what is actually happening inside that small pouch?
That little bag is, in fact, a “chemical factory in your pocket.” This time, we’re going to unlock the secrets of this factory with a hands-on experiment: making your own disposable heat pack to truly feel the heat of chemistry.
【This article is also available on the radio】

When I conduct this experiment in class, the students’ eyes light up. The classroom is filled with exclamations like, “It’s really getting warm!” and “Look, there’s steam coming off it!” The moment an invisible chemical reaction transforms into tangible “heat” that you can touch is the ultimate learning experience that a textbook alone can’t provide.
The DIY Heat Pack Challenge: Materials and Procedure
Our goal is to understand the mechanism of a disposable heat pack and experience the exothermic reaction—how a chemical process called oxidation generates heat. Let’s get started with the preparation!
■ Materials Needed (Per group)
- Iron Powder (300 mesh): approx. 4g (the same one used in the hydrogen sulfide experiment). This is the star of the show!
- Activated Carbon: approx. 2g. The vital supporter that helps the iron react.
- Saltwater (3% solution): 3 cm³. The magic liquid that speeds up the reaction.
- Paper Cup: 1 (small size)
- Thermometer: 1
- Glass Rod: 1
- Weighing Paper: appropriate amount

■ Experiment Procedure
① Put 4g of iron powder and 2g of activated carbon into the paper cup, and mix thoroughly with a glass rod until the powders are uniform.

② Add 3 cm³ of saltwater and quickly stir immediately with the glass rod. The chemical reaction is starting!
③ Insert the thermometer and record the temperature every minute. Measure for 10 minutes and try to plot the change on a graph.
Tip: Stir occasionally to introduce air (oxygen). This will help the temperature rise more effectively.

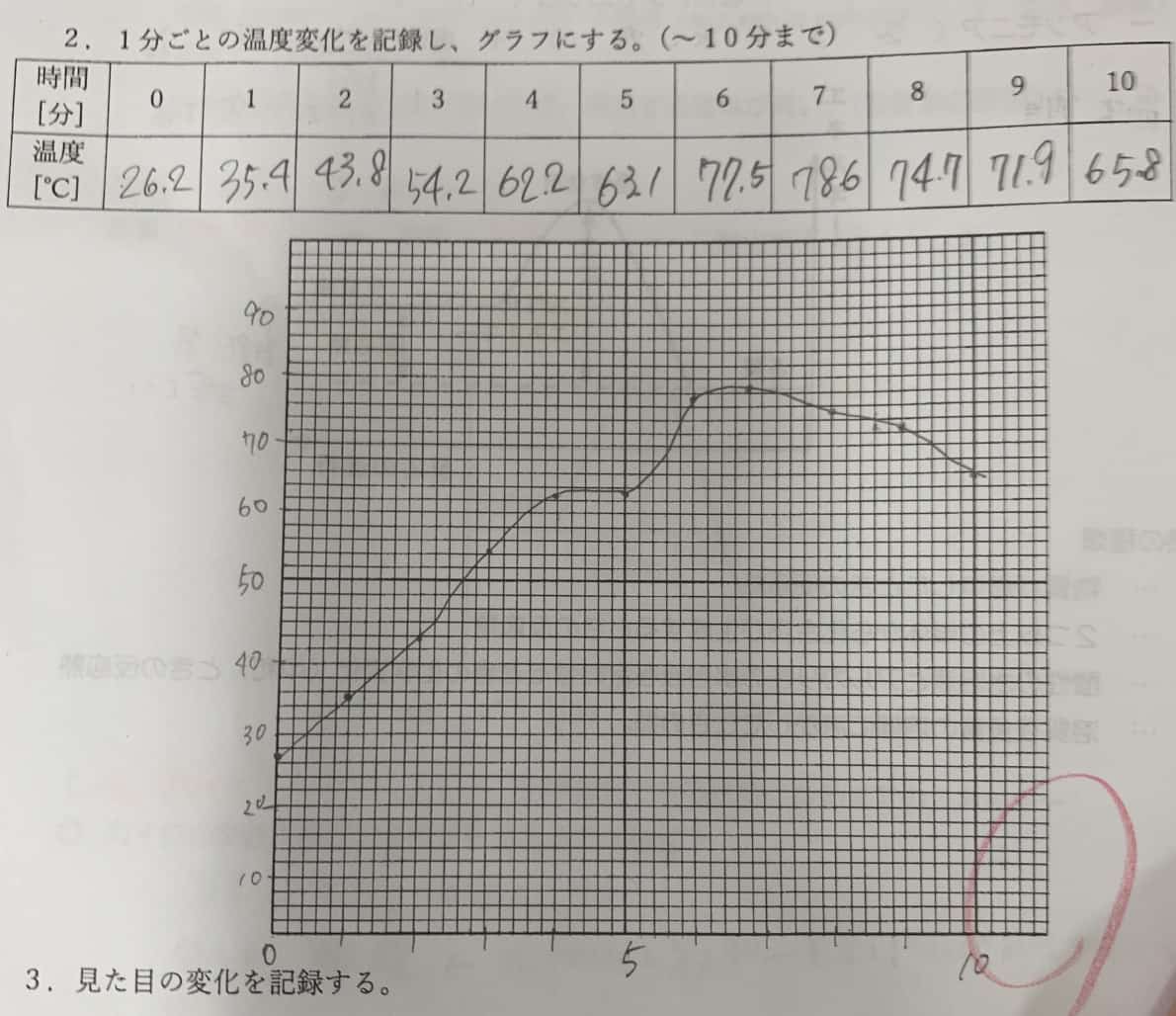
80°C Just by Mixing!? Unraveling the “Heat” of Chemistry
In this experiment, some groups managed to raise the temperature to nearly 80°C, creating so much heat that steam was visible! This is proof that “iron oxidation”—in other words, the “rapid rusting of iron”—is taking place inside the paper cup. Iron has the property of releasing heat when it combines with oxygen from the air to form rust. The secret to your heat pack’s warmth lies in this chemical reaction.
4Fe + 3O₂ + 6H₂O ⟶ 4Fe(OH)₃ + Heat Energy
This chemical equation might look daunting, but think of it as the story of the “All-Star Team” inside your heat pack:
- Iron Powder (Fe): The Protagonist. The source of energy that releases heat by combining with oxygen.
- Oxygen (O₂): The Partner. Comes from the air to react with the iron.
- Water (H₂O) and Salts: The Supporters. They act as catalysts, accelerating the iron-oxygen reaction at breakneck speed. The temperature surge the moment you add saltwater is thanks to them.
- Iron Hydroxide (Fe(OH)₃): The Product. A type of “rust” formed when iron, oxygen, and water combine. Heat is generated as this substance is created.
And commercial heat packs have even more clever engineering built into them:

- Activated Carbon: The Super Assistant. Its surface is covered with countless tiny pores that absorb a large amount of oxygen from the air. It plays the crucial role of gathering oxygen around the iron to sustain and efficiently drive the reaction.
- Vermiculite, Wood Powder, Superabsorbent Polymer: The Regulators. They enhance water retention to prolong the reaction and help distribute the generated heat uniformly throughout the pack. Thanks to them, the heat pack doesn’t get too hot too fast and can maintain warmth for hours.

Safety Promise for a Fun Experiment
This experiment is relatively safe, but the iron powder gets hot while oxidizing. DO NOT throw the cup in the trash until the reaction has finished and the mixture has cooled completely. After the experiment, store the paper cup in a safe place and dispose of it according to the teacher’s instructions on the following day. A simple disposable heat pack is packed with so much scientific ingenuity and cleverness. When you ask “Why?” about the things around you, the world is bound to become much more interesting.
Inquiries & Requests
Let’s make the wonders and fun of science more accessible! I’ve put together easy-to-understand tips and fun science experiments you can do at home. Feel free to search around!
・About the administrator, Ken Kuwako: click here
・For various requests (writing, lectures, experiment classes, TV supervision/appearances, etc.): click here
・Article updates are posted on X!
![]() Experimental videos are streaming on the Science Channel!
Experimental videos are streaming on the Science Channel!
2月のイチオシ実験!梱包材で遊ぼう!
- 静電気の時期になってきました。子供と一緒に梱包材で盛り上がろう!→ やめられなくなる!静電気実験20
 体中に梱包材をはりつけてみよう!
体中に梱包材をはりつけてみよう!
テレビ番組等・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師等・ショー・その他お知らせ
- 2/20(金)「生徒の進学希望実現支援事業」研究授業@福井県立若狭高等学校 講師
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) サイエンスショー予定
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。