The Perilous Promise of ‘Iron and Sulfur’: 13 Crucial Safety Steps for a Middle School Science Experiment
Hi, I’m Ken Kuwako, a Science Trainer. My life is one big experiment.
[This article is also available on a podcast!]

Mixing iron and sulfur and heating them to create iron(II) sulfide—it’s an experiment in sulfurization that every science teacher has likely done at some point. Living in a volcanic country like Japan, it’s worthwhile to experience the smell of hydrogen sulfide. In fact, some hot spring towns have that “rotten egg” smell as part of their daily life. For me, as someone from Gunma Prefecture, it’s a scent that brings back a sense of nostalgia.
However, this experiment has led to accidents reported nationwide every year. If we’re not careful, it could be banned across the country. A simple search for “hydrogen sulfide news” brings up numerous terrifying accident reports. The most critical issue is how the hydrogen sulfide is produced. Teachers are probably giving very cautious instructions, but students who aren’t listening might add too much hydrochloric acid. If an accident happens, the teacher is held responsible. What’s more, there are many subtle details to this experiment that, if overlooked, can lead to serious trouble, difficult conversations with parents, and the loss of a valuable learning opportunity.

6 students hospitalized after hydrogen sulfide incident during experiment. Why is this experiment still necessary despite repeated accidents?
Source: TV Asahi News (May 13, 2025)
When heated, the iron and sulfur inside the test tube react and glow red. This beautiful change creates a memorable “science experience” for students. And best of all, a lesson that uses the sense of smell is more impactful and can drive home a life-saving piece of knowledge: that hydrogen sulfide is a dangerous gas. That’s why it’s crucial to perform this experiment correctly, safely, and with the utmost care. In this article, I’ll share 13 key safety points that I review every year before teaching this lesson. I’ve also included the safety instruction slides I show to my students, so feel free to use them.
[Click here for the instructional slides I use in my classes.]
[I also created a podcast to help remind students about safety. You can listen to it here.]
Listen to the audio file here (MP3)
Before running this experiment, I always do a few practice runs myself. I’ve been fortunate to get through the lessons without any major accidents, but I’m always aware of the risks.
The methods I’ve written about here are just one example, focused on prioritizing safety. Different textbooks may suggest other ways, such as using steel wool. Please adapt these methods to best suit your school’s equipment and teaching setup.
I also get a lot of inquiries asking if the photos and slides can be used for classes. You are free to use any of the content posted here. I hope it helps you create safer and more effective lessons.
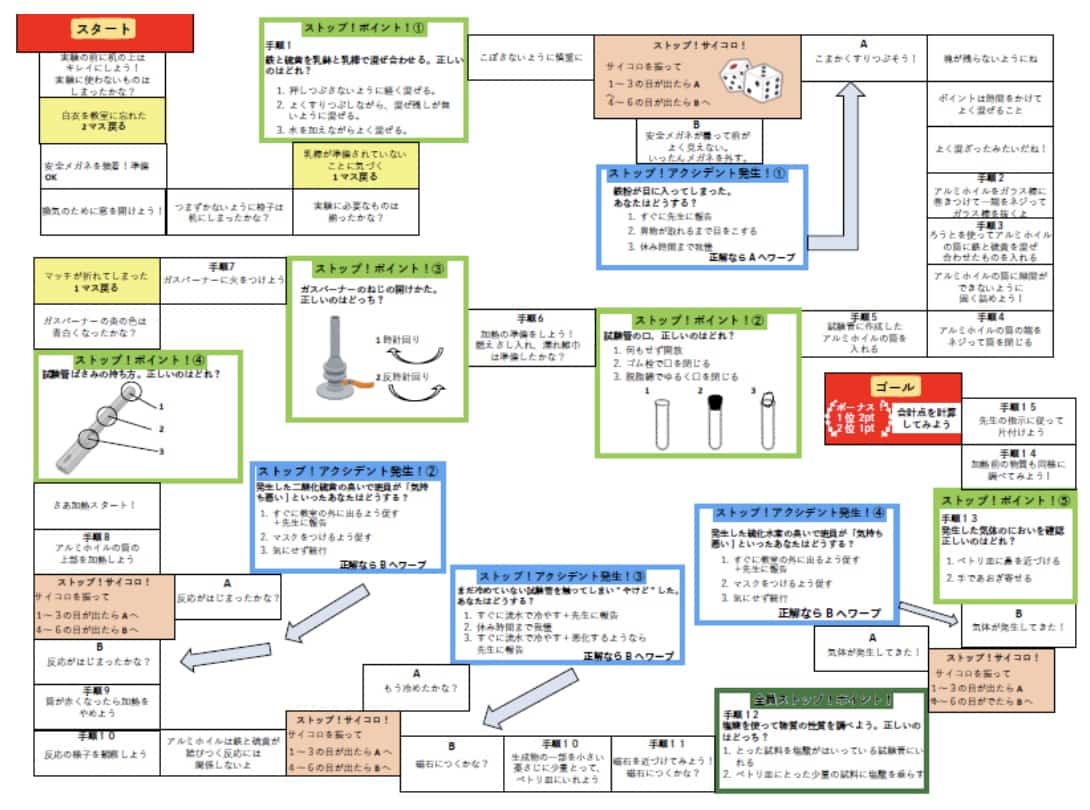
I also helped develop an educational game about the iron and sulfur experiment, called “Iron and Sulfur Sugoroku,” with my colleague Hina Morishige. It’s a great way for students to learn correct procedures while having fun. It’s especially effective when played before the experiment. Please give it a try! It’s available for free download.
The Experiment
Step 0: Get the word out and turn on the ventilation!
It’s a good idea to let the school nurse and homeroom teacher know you’ll be doing the experiment, just in case. Tell your students to bring their lab coats and safety goggles.
When things get busy, you might remember to open the windows but forget to turn on the exhaust fan.
Safety Point 0: Notify the school nurse and homeroom teacher in advance.
Safety Point 1: Turn on the exhaust fan at the very beginning so you don’t forget when things get hectic.

Step 1: Mix the iron and sulfur powders (7.0g iron, 4.0g sulfur)
As a teacher’s pre-lab preparation, you need to weigh out 7.0g of iron powder and 4.0g of sulfur powder in advance (since there isn’t enough time for students to do this in a 50-minute class). The amount is important; if you have too much sulfur, it’s more likely to produce sulfur vapor. This step is a hassle every year. I’d love to have the students do it, but it takes too long and eats into the experiment time. This year, I got help from the science club members. The iron powder should be 100 mesh or finer, as a 60-mesh powder might not react properly. I used 300-mesh powder this time.

Safety Point 2: The iron powder should be 100 mesh or finer!
You can find it on Rakuten here:
Iron Powder 300 Mesh [Shipping not available to Hokkaido or Okinawa]

Pour the iron and sulfur into a mortar and mix them well. I have a separate mortar for iron(II) sulfide and one for copper oxide. This makes it easy for the next class to use them without needing to be washed.

Mix it thoroughly. Keep mixing until there are no clumps of sulfur left and the mixture is a uniform gray color.
Safety Point 3: Mix thoroughly. If the chemical reaction isn’t complete, sulfur vapor will be released.
FYI: The melting point of sulfur is 112°C, and its boiling point is 446°C. A Bunsen burner flame is around 1500°C.

This is an example of a poorly mixed sample. You can still see yellow clumps of sulfur. You should keep mixing until the entire mixture is gray.
Step 2: Make two aluminum foil tubes and fill them with the sample.

The aluminum foil should be about 5 cm x 10 cm and prepared beforehand. Use two AAA batteries taped together as a core to wrap the foil around. Press down firmly on the desk to make a tight roll.
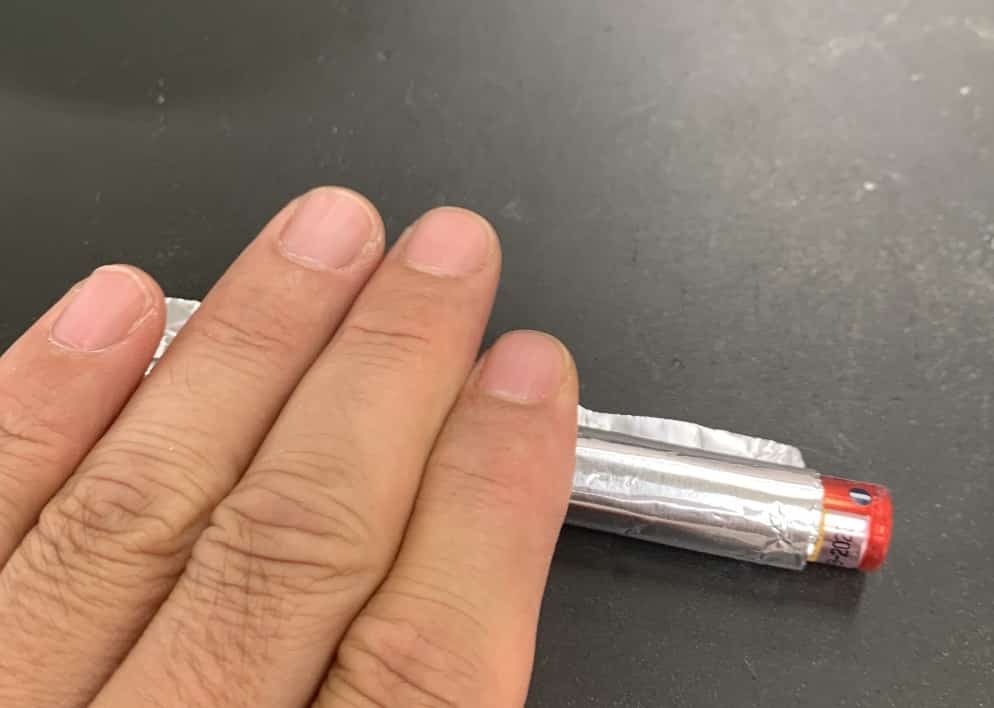
Pinch one end shut and pull out the batteries. You need to make two of these tubes.

Transfer the mixture onto weighing paper.

Then, use a funnel to pour the mixture into the tubes.

Once it’s in, gently tap the tube on the desk to settle the powder and ensure good contact. Warn students not to tap too hard, or the foil might tear. Tell them to tap lightly. Also, you can tell them that the funnels don’t need to be washed and can be reused by the next class.
Step 3: Put the sample in a test tube and heat it.
Take the two samples, A and B. Keep B as your control, and put sample A into a test tube. Cap the top of the test tube with a wad of cotton, then heat the top of the aluminum foil tube. Once the reaction starts, stop heating and observe. The reaction should begin in about a minute.

Safety Point 4: Cap the test tube with cotton. This prevents toxic vapors like sulfur vapor from escaping.

Heat the top part of the aluminum foil tube, not the bottom. The reason is that the heat from the reaction combined with the heat from the Bunsen burner can sometimes crack the test tube, causing the bottom to fall off if heated from below. Heating from the top also has the benefit of keeping the test tube from getting dirty, so it can be reused for the next class. (I was able to reuse the same test tubes for four classes).
Safety Point 5: Heat the top part of the aluminum foil tube.
Safety Point 6: Hold the test tube with the test tube clamp near the top. Never hold the scissors part of the clamp, as it could accidentally open.

Once the reaction starts, you can remove the Bunsen burner flame. The reaction will continue on its own. It’s a beautiful, slow-burning reaction, like fireworks.

Safety Point 7: Keep the Bunsen burner on so students can focus on the chemical reaction. This is one of the key moments of the lesson.
Safety Point 8: Remind students to keep their hands away from the bottom of the test tube.

Let the test tube cool down a bit by holding the test tube clamp in your hand, then place it on the empty mortar to cool completely.

While you wait for it to cool, have students put away the heating equipment.
Step 4: Once fully cooled, remove sample A from the test tube and compare its properties to sample B.
Touch the tip of the test tube with a damp cloth. If it doesn’t sizzle, wrap the entire test tube in a damp cloth to cool it completely. Remove the cotton and take the sample out.

Bring a magnet close to it.

Safety Point 9: A ferrite magnet will work well, but a neodymium magnet might attract the iron(II) sulfide as well. However, the attraction will be much weaker. Point out the difference in attraction to students.
Step 5: Take a small piece (1-2mm) of the sample, place it in a Petri dish, add three drops of hydrochloric acid from a dropper bottle, and waft the smell toward your nose.
This is the most dangerous part and where most accidents happen! Double-check the ventilation, open the windows, and open the classroom doors at the front and back. Emphasize and repeat the instructions to only use a “small piece” and “three drops” of hydrochloric acid. Students who aren’t listening might dump a large amount of acid on the sample.
Using a dropper bottle prevents students from adding too much. But even with these, some students may try to open the bottle by twisting the cap. It might be safer for the teacher to add the acid themselves, being extra careful!

Safety Point 10: Open doors and windows for ventilation. Also, break the iron(II) sulfide into a small piece before adding the acid. Use diluted acid (3%) in a dropper bottle and instruct students to add only three drops.
Safety Point 11: Tell students that if they feel unwell, they can leave the room. Since the gas is heavier than air, tell them to do the experiment while standing (this is usually the default anyway).
Safety Point 12: The gas is toxic, so tell students to gently waft the smell toward their nose with their hand. Do not put their face directly over the dish or inhale deeply. Once everyone has smelled it, instruct them to add a small amount of water to stop the reaction.
You can also place the piece of iron(II) sulfide in a test tube to add the acid and smell it that way, but I found the Petri dish method to be more effective. Since hydrogen sulfide is heavier than air, it takes a while to fill a test tube and reach the opening. I worried that students might get impatient and put their noses right up to the test tube to smell it, which would be dangerous. That’s why I recommend the Petri dish method.
Instruct students to wash the test tubes with a brush. They won’t get perfectly clean. Collect all leftover iron(II) sulfide and any other waste and dispose of it in the non-burnable trash. Do not pour it down the sink. You can also check the test tubes for cracks, and if they’re not cracked, they can be reused even if they’re a bit stained.
Safety Point 13: Collect all remaining materials. Do not pour them down the drain. Teachers must be careful when disposing of the waste.
After collecting the leftover materials, the teacher needs to be careful when disposing of them. There was a report of a fire starting in a science lab, and based on the timing, it’s possible the fire was caused by the mixture of iron and sulfur igniting. The truth remains unknown.
Alternative Methods (For Reference)
You can also heat the mixture directly without a test tube. I did this for a few years. The reaction is very visible and great for observation. However, students tend to get too close, which can lead to them inhaling sulfur vapor and coughing. For a safer experience, I recommend the cotton-plug method described above.
Some textbooks also describe a method using sulfur and steel wool in a test tube. This is what that looks like:



Related Resources
[Click here for the instructional slides I use in my classes.]
[I also created a podcast to help remind students about safety. You can listen to it here.]
Listen to the audio file here (MP3)
Contact & Inquiries
Let’s make science fun and accessible! I share simple, engaging science experiments you can do at home, along with tips and tricks. Feel free to explore and search for more articles!
・About the author, Ken Kuwako, click here.
・For all inquiries (writing, lectures, science workshops, TV supervision, etc.), click here.
・Get article updates on X!
![]() The Science Channel has experimental videos!
The Science Channel has experimental videos!