The Cloud Maker: The Hidden Science of Your Soda Saver
Hi, I’m Ken Kuwako, your Science Trainer. Every day is an experiment.
That fizzy carbonated drink after a sports practice or on a hot day is simply the best! But everyone has had that disappointing experience: you put an opened bottle back in the fridge, take a sip the next day, and think, “…Ugh. It’s just sweet water.”
To prevent this sad tragedy, there’s a clever gadget called the “Soda Fresh” or “Shuposhupo-kun” (a nickname meaning ‘pump-pump guy’). This brilliant invention attaches to the top of the bottle, allowing you to pump air in and increase the internal pressure, which stops the carbon dioxide gas from escaping the liquid.
Soda Fresh (Amazon)
But did you know this handy tool is much more than just a soda preserver? It’s actually a powerful science machine that can create a “cloud” right inside the plastic bottle! Today, we’re diving into the dynamic scientific secret behind this “Shuposhupo” device: why it generates heat and how it allows you to make your own cloud.
What’s Really Happening Inside the “Shuposhupo”? — The Moment Pressure Turns into Heat
First, let’s try an experiment: use the device to pump (pressurize) air into a plastic bottle. If you place a thermometer strip inside the bottle, and then pump vigorously, you’ll feel the bottle warm up when you touch it. Why does the temperature rise just from adding air?

The reason is simple: you are forcefully pushing air molecules into a confined space. To help you picture this, imagine a packed train car. Just before the doors close, what happens when a station attendant pushes even more people inside (i.e., applies pressure)? Everyone inside gets squished and starts bumping into each other, right?
The exact same thing is happening inside the bottle. The “work” of pumping air in gets converted into energy—in this case, “heat”—by causing the air molecules to move more violently. In physics, we call this process “Adiabatic Compression.” The simple act of preserving your soda actually lets you physically feel a crucial law of physics: the relationship between pressure and temperature.
The Experiment: Creating a “Cloud” in a Bottle!
Now, for the main event. We are going to create a cloud using the opposite phenomenon of Adiabatic Compression: “Adiabatic Expansion.” The key is to pay attention to how the temperature changes when air rapidly expands.
What You’ll Need:
- An empty, strong plastic bottle (designed for carbonated drinks, 500ml recommended)
- Soda Fresh (or any pressurizing pump)
- A small amount of water
- An incense stick (or mosquito coil) *Caution: Handle fire safely!
Experiment 1: Water Only (A Little Underwhelming…?)
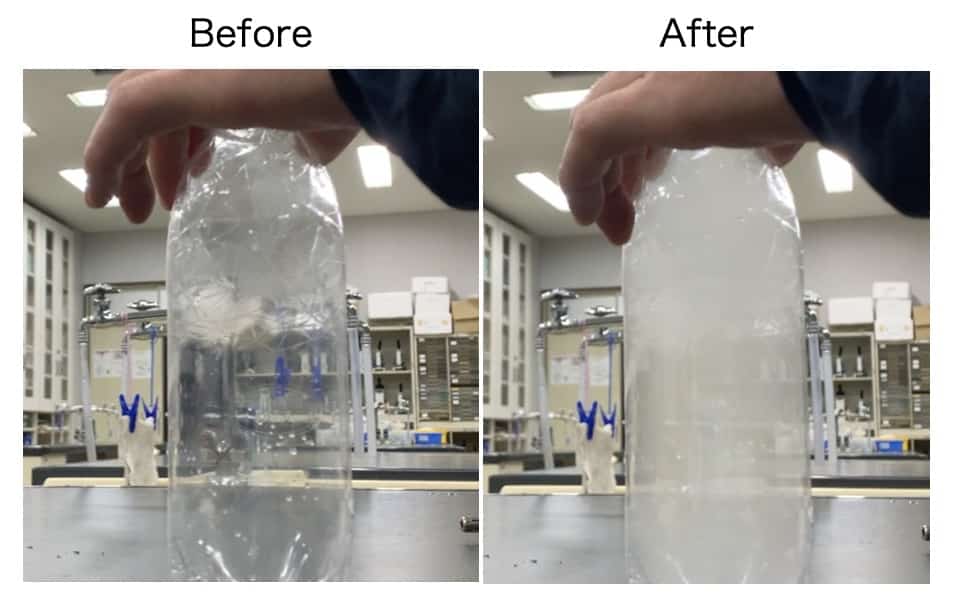
First, let’s try the experiment using only water and air.
- Pour a small amount of water into the bottle, shake it gently to wet the inside, and then pour the water out. (This raises the humidity inside the bottle to nearly 100%).
- Attach the Soda Fresh and pump with all your strength until the bottle feels very firm. (Shuposhupo!)
- At this point, the temperature inside the bottle has risen. (Warmer air can hold much more water vapor (a gas)!)
- In this pressurized state, remove the lock and quickly open the cap to release the pressure! POP!
- → The air expands rapidly, and the temperature drops suddenly!
- → The rapidly cooled air can no longer hold all the water vapor (it becomes saturated), and the excess vapor turns back into tiny droplets of water, causing a slight mist to appear.
What did you see? To the naked eye, you probably thought, “Huh? Maybe it got a little white for a second…?” Honestly, it’s a bit underwhelming, right? The truth is, you need more than just water vapor and a temperature change to create a proper cloud.
Experiment 2: Adding Incense Smoke (A Real Cloud Appears!)
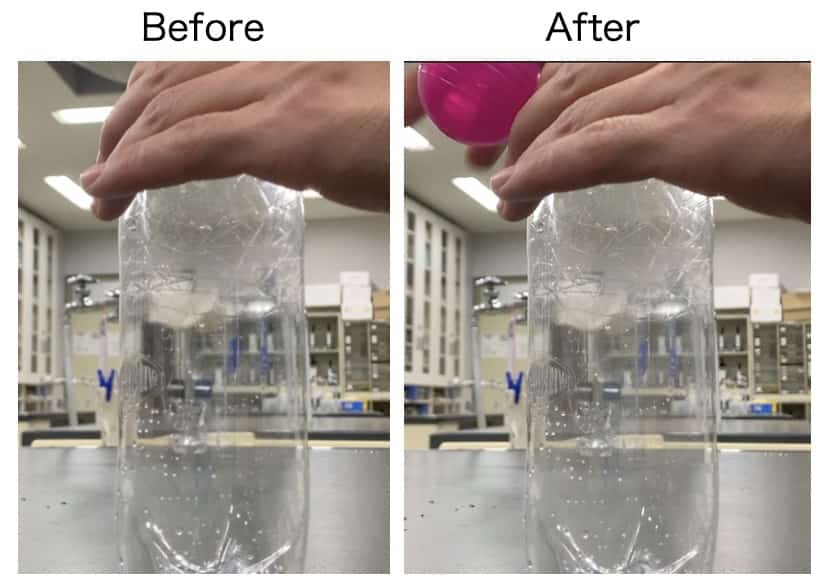
Next, let’s add the magic ingredient. Put just a tiny puff of incense smoke (a quick moment is all you need!) into the bottle, and then follow the exact same procedure (Steps 1-5) as before.
And the result…
→ This time, the inside of the bottle turns completely white—a distinct “cloud” has formed!
The only difference was the presence of smoke. Why such a dramatic change? The answer is that the incense smoke acted as a core, or “Condensation Nucleus.” In Experiment 1, the cooled water vapor (a gas) was lost, thinking, “I want to turn back into water, but I don’t have anywhere to start…” But in Experiment 2, the tiny smoke particles (aerosols) provided a “place to gather.” The water vapor instantly rushed to these particles and was able to change (condense) into visible “water droplets (a cloud).”
The Small Bottle is a Model of Earth’s Sky
This experiment isn’t just a fun trick. For students, it leads to a deeper understanding of global meteorological phenomena.
- Understanding the True Nature of Clouds: Many people think clouds are just fluffy “water vapor (a gas),” but this experiment demonstrates that they are actually masses of tiny water or ice particles (liquid/solid).
- Understanding Temperature, Pressure, and Weather: When air warmed by the sun rises and expands (depressurizes), its temperature drops, and clouds form. What happens inside this bottle is the exact same cloud-formation mechanism that occurs high in the atmosphere.
- Realizing the Importance of the Invisible: The most amazing discovery is realizing that “invisible dust particles (aerosols) in the air are essential.” The real sky is also filled with fine, unseen particles—dust, soot, and even sea salt crystals—and without these “nuclei,” clouds simply cannot float in the sky.
An everyday tool designed to keep your soda fizzy can actually become a miniature device that recreates Earth’s atmosphere. Realizing how “seemingly unrelated things are connected” is the true joy of studying science. We encourage you to try this at home!
Inquiries and Services
Make the wonders and fun of science more accessible! We provide easy-to-understand explanations of fun science experiments you can do at home, along with tips and tricks. Feel free to search around! *The contents of this science blog have been published in a book. Find out more here
*About the Author, Ken Kuwako, click here *For Inquiries (Writing, Lectures, Experiment Workshops, TV Supervision/Appearances, etc.), click here *Article updates are available on X!
![]() We post experiment videos on the Science Topics Channel!
We post experiment videos on the Science Topics Channel!
2月のイチオシ実験!梱包材で遊ぼう!
- 静電気の時期になってきました。子供と一緒に梱包材で盛り上がろう!→ やめられなくなる!静電気実験20
 体中に梱包材をはりつけてみよう!
体中に梱包材をはりつけてみよう!
テレビ番組等・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師等・ショー・その他お知らせ
- 2/20(金)「生徒の進学希望実現支援事業」研究授業@福井県立若狭高等学校 講師
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) サイエンスショー予定
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。



