Musical Chairs of Metals! Who Reigns as the Ion King? A Microscale Experiment on Metal Reactivity
I’m Ken Kuwako, your Science Trainer. Every day is an experiment.
Why does an iron nail rust, but a gold ring keeps its shine forever? How can a simple combination of metals in a battery generate electricity? The secret lies in the metals’ strong desire to become “Ions!“—in other words, their “Tendency to Form Ions.”
Today, I’m introducing an eco-friendly experiment that can be performed on a micro-scale (super miniature size) to reveal the invisible “commitment” of these metals! The key features are “Minimal Reagents, Great Visibility, and Safety.” It’s easy for middle school students to grasp, and the minimal preparation is a bonus. Students who love chemistry, and even those who find it a bit challenging, are guaranteed to be thrilled by the color changes.
The concept of “metals becoming ions in an aqueous solution” might seem abstract at first. However, by witnessing dramatic changes like the metal’s color shifting or the solution’s blue color disappearing, students will have that “Aha! I get it now!” moment, leading to a deeper understanding. Furthermore, using microplates and mini-bottles drastically cuts down on liquid waste, minimizing the experiment’s environmental impact.
In this article, we’ll walk you through the preparation, observation, and the all-important post-experiment analysis of “Why did this happen?”
What You’ll Need (Materials)
Tools & Reagents | Description — | — Copper plate, Zinc plate, Magnesium plate | Our main cast: three metal players
- 3% Copper Sulfate Solution | Copper ions (Blue)
- 3% Zinc Sulfate Solution | Zinc ions (Colorless)
- 3% Magnesium Sulfate Solution | Magnesium ions (Colorless)
 💡 Recommended Item: The Petit Bottles from Narika (link) offer far better control, allowing you to dispense reagents one drop at a time, making them vastly superior to 100-yen shop alternatives.
💡 Recommended Item: The Petit Bottles from Narika (link) offer far better control, allowing you to dispense reagents one drop at a time, making them vastly superior to 100-yen shop alternatives.

https://www.rika.com/product/detailed/S75-1140-02
Other necessary items include a microplate (a plate for observing reactions with small amounts of liquid), tweezers, and safety goggles.
Reagent Preparation (How to Make 3% Solutions)
Here are the instructions for preparing the reagents. This is a slightly technical note for teachers.
To make 3% Copper Sulfate Solution: Dissolve 4.9g of Copper(II) Sulfate Pentahydrate (CuSO4・5H20) in 100g of water.
To make 3% Zinc Sulfate Solution: Dissolve 5.6g of Zinc Sulfate Heptahydrate (ZnS04・7H20) in 100g of water.
To make 3% Magnesium Sulfate Solution: Dissolve 6.5g of Magnesium Sulfate Heptahydrate (MgSO4・7H20) in 100g of water.
Experiment Procedure
Metal Plate Preparation
Lightly sand each metal plate to make the surface shiny. This removes the oxidized film (like rust) and reveals the metal’s “true nature.” This dramatically increases the likelihood of a reaction.
Placement in the Microplate
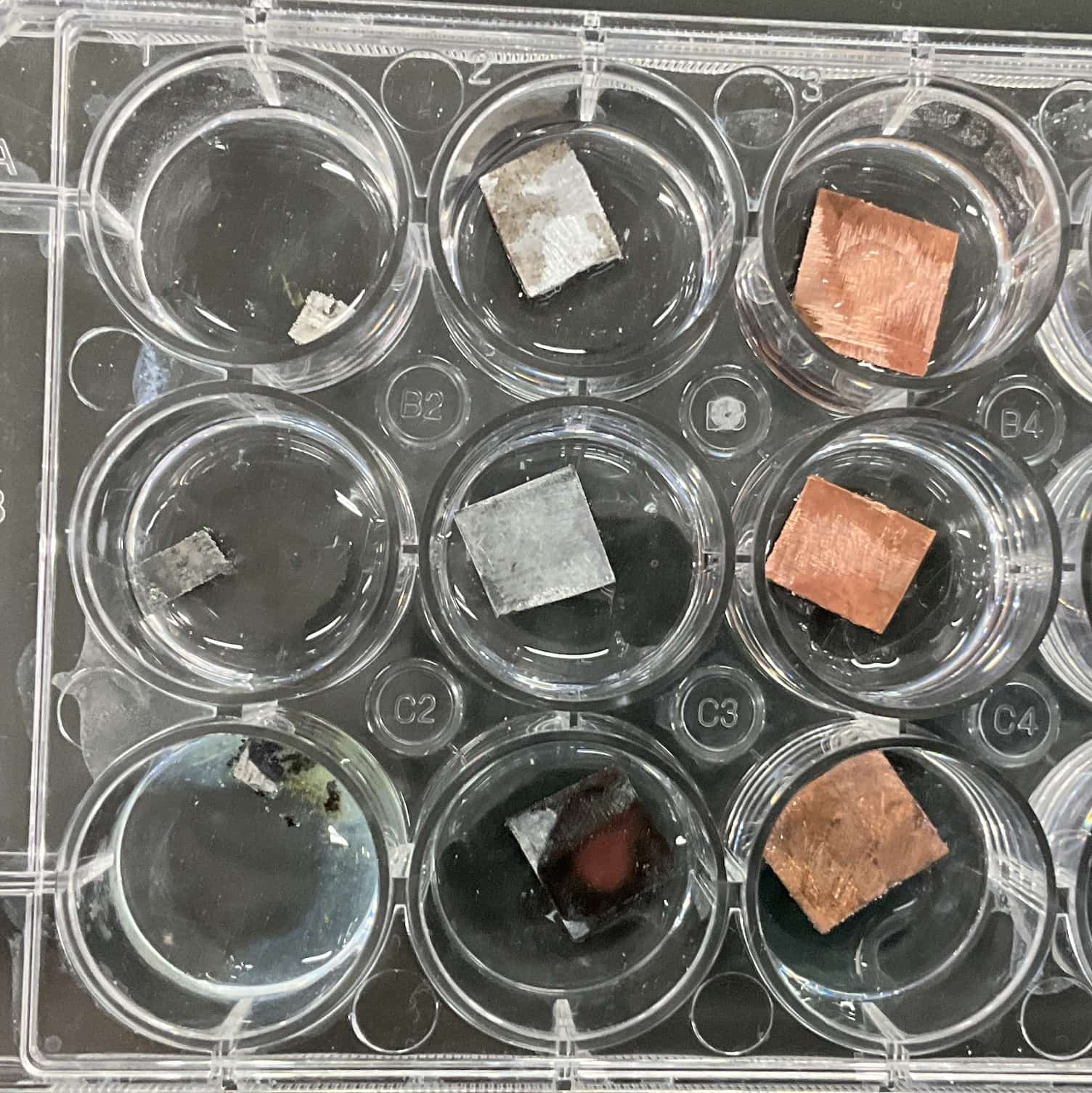
Place the three types of metal pieces into the cells (small wells) of the plate. (We will use 9 cells to test all possible combinations: 3 types of metals × 3 types of solutions).
Drop the Solutions Use the mini-bottles to add about three drops of the corresponding solution to each metal piece.
Observe the Reaction Pay attention to three key points: color change, bubble formation, and the appearance of the metal surface.
Experiment Results and Observations
Here are the results from the actual experiment.
This is what the cells looked like. Out of the total 9 combinations, clear reactions were observed in a few cells (such as the bottom three cells on the left in the image).
The most dramatic changes were seen with the metals placed in the blue Copper Sulfate solution.
【Copper Sulfate Solution × Magnesium Plate】
 Left: Before reaction, Right: After reaction
Left: Before reaction, Right: After reaction

The magnesium chip has become slightly thinner (it has dissolved).
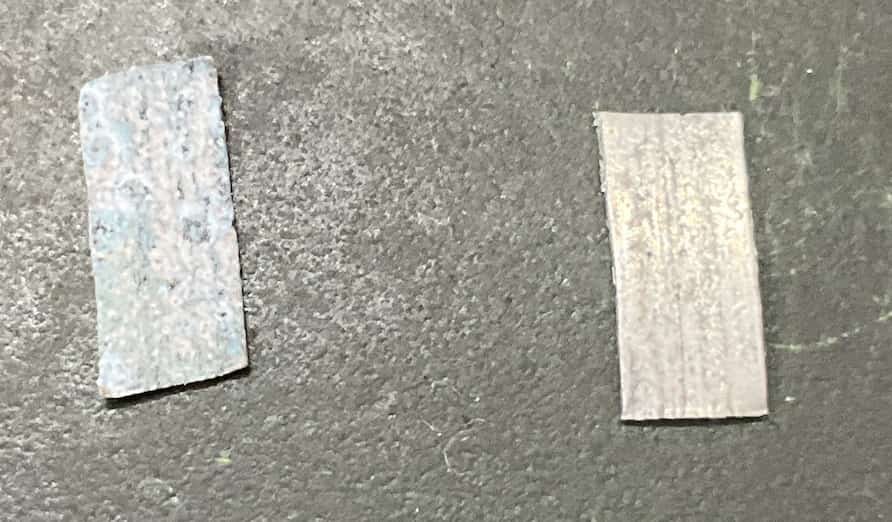
Right is new, Left is after the reaction. The surface has turned black.
When the magnesium was added, its surface immediately turned black, and the blue color of the solution faded! The magnesium plate itself also slightly dissolved (became thinner).
【Copper Sulfate Solution × Zinc Plate】
 Left: Before reaction, Right: After reaction
Left: Before reaction, Right: After reaction The zinc plate also developed a reddish deposit on its surface. While not as vigorous as the magnesium reaction, a clear reaction took place.
The zinc plate also developed a reddish deposit on its surface. While not as vigorous as the magnesium reaction, a clear reaction took place.
【Copper Sulfate Solution × Copper Plate】
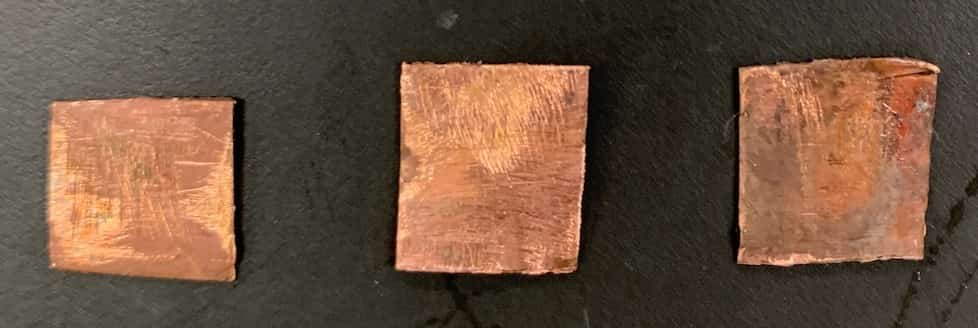 The copper plate showed absolutely no change when placed in the copper sulfate solution.
The copper plate showed absolutely no change when placed in the copper sulfate solution.
In summary, the observed changes for each combination were:
Copper Sulfate × Magnesium: Metal surface turns black, precipitate appears. Copper Sulfate × Zinc: Reddish deposit on the metal surface. Copper Sulfate × Copper: No change.
Analysis: The Tendency of Metals to Form Ions (Reactivity Series)
From these reaction results, we can deduce the order of metals’ tendency to release electrons and form ions (the Reactivity Series):
Magnesium > Zinc > Copper
Magnesium reacted the most vigorously, while copper showed no change at all. This demonstrates that magnesium readily releases electrons and is easily ionized.
Summary and Classroom Application Tips
This micro-scale experiment is a trifecta of space-saving, low-cost, and safety. The dramatic color changes—like the blue fading or a red metal appearing—make the chemical change tangible and easy to grasp. The analysis process naturally unfolds into a “Why?” story, making it perfect for group work. Since the liquid waste is only a few drops per microplate cell, cleanup is simple and environmentally friendly!
Understanding the reactivity series is a crucial foundation, connecting to topics like the “Daniell cell” (a device that generates electricity by utilizing the difference in the reactivity of zinc and copper), taught in middle school science, as well as “Corrosion (rusting) of Metals” in high school chemistry.
Let’s explore the fun of chemistry together with this engaging experiment!
Micro-Scale Experiments on Amazon
Inquiries and Requests
Bring the wonder and fun of science closer to you! We’ve compiled easy-to-understand fun science experiments you can do at home, along with tips and tricks. Feel free to browse around!
・About the Operator, Ken Kuwako: Click here ・For various requests (writing, lectures, experiment classes, TV supervision, appearances, etc.): Click here ・Updates on articles are posted on X!
![]() We post experiment videos on the Science Channel!
We post experiment videos on the Science Channel!
3月のイチオシ実験!
- 押し花を作ろう!:梅や桜の花の押し花を作ってみましょう。特別なケースに入れると、長く保存できて、しおりにもなります。
テレビ番組・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
- 2月27日放送予定「チコちゃんに叱られる」(NHK)の科学監修しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師・ショー・その他お知らせ
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) 秘密兵器「帯電ガン」が炸裂!ビリビリ!ドキドキ!静電気サイエンスショー@千葉市科学フェスタ(午後予定)
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。



