A Tiny Universe in a Petri Dish: Discover the Magic of Diffusion with Coffee Sugar in Just 10 Minutes!
I’m Ken Kuwako, your Science Trainer. Every Day is an Experiment!
Have you ever gently dropped a sugar cube into a cup of tea? Even without stirring, a ripple of amber color slowly, yet surely, spreads outward…
You might think, “Well, it’s dissolving—what’s the big deal?”
But hidden within that seemingly “obvious” process is an epic drama of the microscopic world, completely invisible to the naked eye. Today, I’m going to introduce a quietly beautiful science experiment starring just a single sugar cube. It’s the perfect way for junior high students, especially those new to the world of “aqueous solutions,” to grasp its fascination and depth. The setup is surprisingly simple. Come on, let’s open the door to the unseen world together.
The Silent Dance of Color Begins
The stars of this experiment are a Petri dish, a single coffee sugar cube, and your focused gaze. When you place the sugar cube in the water inside the dish, the color starts to spread out, little by little, as if by magic. No one is stirring, yet it spreads uniformly and peacefully, almost as if it has a will of its own.
Your students are bound to exclaim: “How can it spread without being stirred?” This simple, pure sense of wonder is the gateway to all science. It’s the perfect catalyst for them to begin imagining the invisible world of “particles.”
Experiment Setup
Petri dish (A transparent, flat-bottomed one is best for observation)
Coffee Sugar Cube (A colored, slowly dissolving coarse sugar, like brown sugar crystals or a coffee sugar cube, works best)
Water (Room temperature is fine. Just enough to cover the bottom of the Petri dish)
100mL Beaker (Used to pour the water gently)
Measuring Sheet (With concentric circles to record how far the color spreads)
*The measuring sheet is placed underneath the Petri dish. You can certainly make your own, but I have prepared a PDF for you to use if you like.
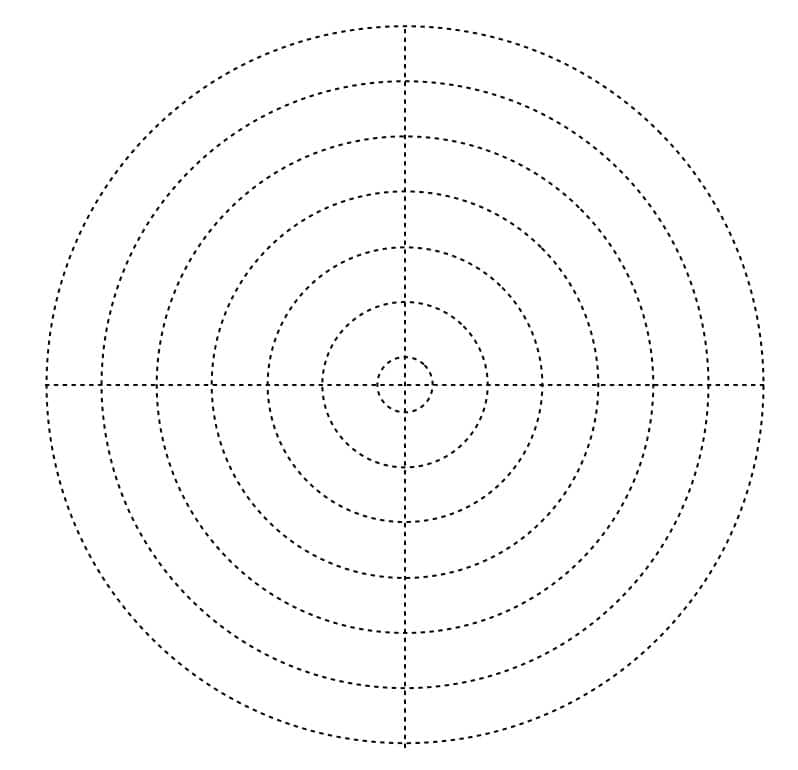
Concentric Circles (For observing how the coffee sugar dissolves) (PDF)
How to Observe and Class Flow
- Gently pour water into the Petri dish (just enough to barely cover the bottom).
- Once the water has completely stopped moving, softly drop the coffee sugar cube into the center.
- This is the most critical step! DO NOT stir, DO NOT shake, and watch with bated breath.
- Record how far the color has spread on the measuring sheet’s scale every 2 minutes for a total of 10 minutes.
The time spent simply and quietly observing the change itself leads to profound learning. Seeing is believing. First, please watch this video:
Clues to the Unseen World: Getting to the Core of Science
Now, for the main event—the science behind the magic. This phenomenon is called Diffusion. Although invisible, the particles (molecules) of water, and the particles (molecules) of sugar, are not actually standing still. They are constantly vibrating and zipping around. This motion is called Thermal Motion.
Inside the Petri dish, countless water molecules are energetically zooming around like little kids, constantly bumping into the sugar molecules. The sugar molecules, upon being struck, gradually break apart and are pushed in all directions by the water molecules, carrying them away.
Let’s pose these questions to your students:
Why did it spread without being stirred?
→ Maybe the invisible water particles bumped into the sugar particles and carried them away?
Why did it spread equally in all directions (up, down, left, right)?
→ This proves that water particles move randomly, not just in a specific direction—they are truly moving chaotically in every direction!
What do you think would happen to the speed of spreading if we used hot water?
→ The higher the temperature, the more vigorous the movement (thermal motion) of the water particles becomes. Therefore, it should spread much faster!
Through this simple observation, students can experientially and convincingly construct the fundamental scientific model that “particles are constantly in motion.”
“The sugar is just dissolving”—but what is happening in this small Petri dish is governed by the exact same principle, Diffusion, which is responsible for perfume spreading across a room, oxygen being absorbed into the blood in the lungs, and even nebulae expanding in the distant universe. When students feel that the laws of the microscopic world connect to our own bodies and even the cosmos, science is bound to become much more interesting.
Inquiries and Requests
Bring the wonder and fun of science closer to you! I’ve compiled easy-to-understand tips and exciting science experiments you can do at home. Feel free to search around for more!
・About the author, Ken Kuwako: Click here
・For various requests (writing, lectures, science classes, TV supervision, appearances, etc.): Click here
・Updates on articles are posted on X!
![]() Experimental videos are available on the Science Neta Channel!
Experimental videos are available on the Science Neta Channel!
3月のイチオシ実験!
- 押し花を作ろう!:梅や桜の花の押し花を作ってみましょう。特別なケースに入れると、長く保存できて、しおりにもなります。
テレビ番組・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
- 2月27日放送予定「チコちゃんに叱られる」(NHK)の科学監修しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師・ショー・その他お知らせ
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) 秘密兵器「帯電ガン」が炸裂!ビリビリ!ドキドキ!静電気サイエンスショー@千葉市科学フェスタ(午後予定)
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。



