The Secret Science of Melting: Why Your Heating Stops Working (The Hidden Drama of Palmitic Acid)
I’m Ken Kuwako, your Science Trainer. Every Day is an Experiment!
Chocolate melts in your hand. Water turns into a solid block of ice in the freezer. All around us, “changes of state”—where a substance transforms its appearance—happen so naturally we barely notice. But have you ever closely watched what happens to a substance’s temperature right in the middle of that change?
A fascinating scientific drama is actually hidden there. Today, let’s explore the mysterious world of temperature change using a familiar star from school science labs: Palmitic Acid!
What Exactly is Palmitic Acid?
The name “Palmitic Acid” might sound a little unfamiliar. However, it’s actually something very common in our daily lives.
Palmitic acid is a type of nutrient called a “fatty acid,” and as its name suggests (palm oil), it’s the main component of palm oil, which is extracted from oil palm trees. You’ll find it in a wide range of products we consume, as well as in soaps and cosmetics. It has a fun property: at room temperature, it’s a white solid, similar to butter, but when heated, it turns into a clear liquid.
In the world of chemistry, it looks like this (chemical structure formula). Its key feature is a long, thin shape with a chain of Carbon (C) atoms lined up.
Time to Experiment! The Drama Hidden in the Thermometer’s Numbers
Now, let’s start the experiment! The goal is to carefully observe the temperature change as palmitic acid is heated and transforms from a solid to a liquid. We’re going to recreate that “melting curve” you saw in your textbooks, right here with our own hands.
Equipment and Preparation
Here’s what you’d need if you were doing this in a classroom setting:
• Palmitic Acid (a small amount is fine; it’s safe and easy to handle)
• Test tube (or small beaker)
• Thermometer (digital or analog is fine)
• Heating apparatus (hot water bath, hot plate, etc.)
• Timer
• Spreadsheet or graphing paper (for students)
The setup looks like this:

The temperature at which palmitic acid changes to a liquid is its “melting point,” which is 62.9
∘
C (145.2
∘
F). This is where the drama begins.
When you actually start heating it…
• As it nears 60
∘
C, you might notice something: the temperature rise begins to slow down…
• The white solid gradually turns into a clear liquid! All while the temperature barely changes!
• The instant it all turns to liquid, the temperature starts climbing rapidly again!
Everyone is bound to gasp in amazement at the change happening right before their eyes. This is the moment the graph from your textbook becomes a real-life phenomenon. For our experiment, we’ll record the data on a spreadsheet and generate a graph. Here is the link to the recording file.
The Key to Success? Starting with “Warm Water”!
To capture this impressive moment, you actually need a little trick. The success of the experiment hinges on “how you heat it.”
If heated from cold water:
Example:
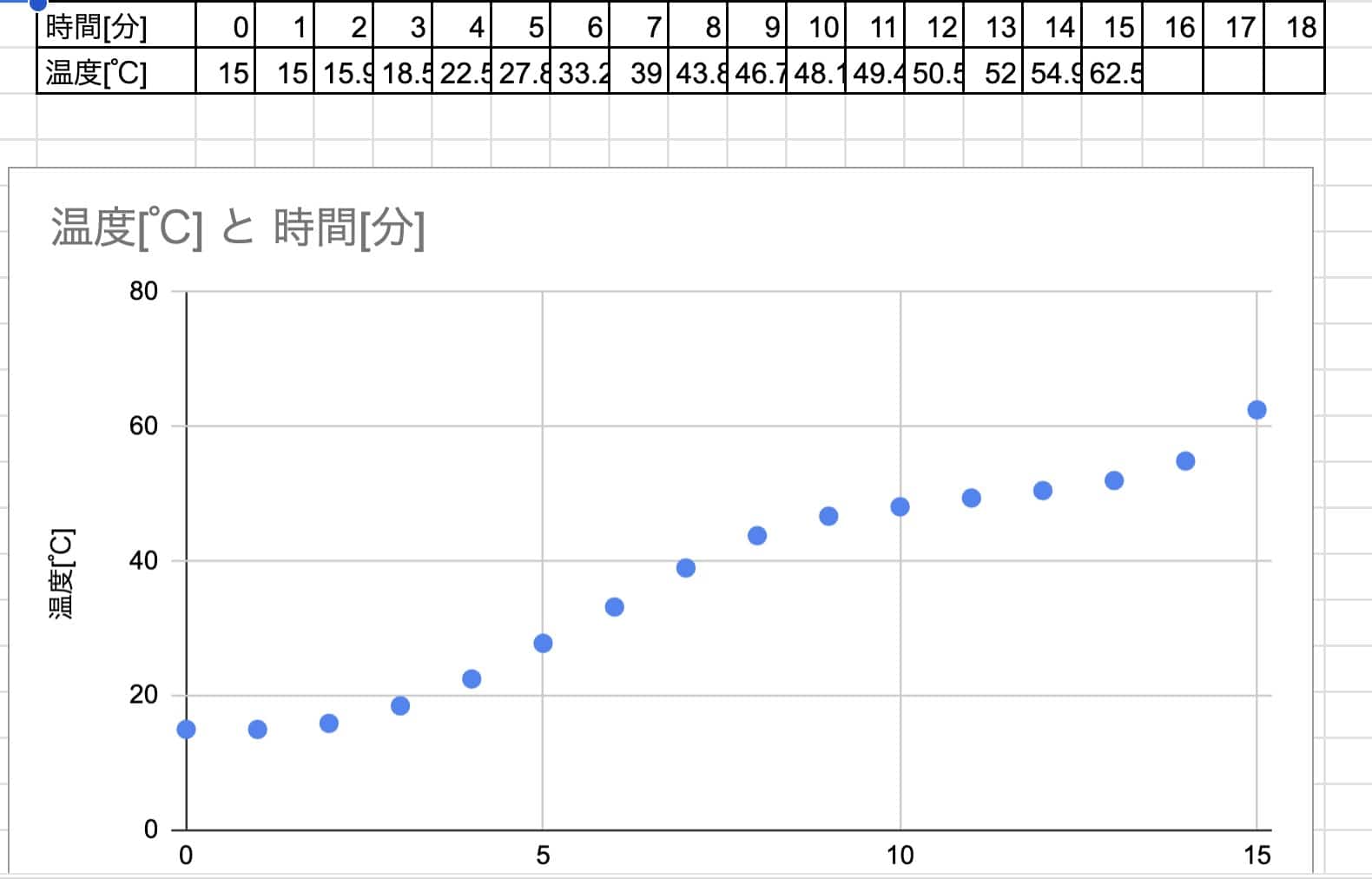
→ Too slow! It didn’t even reach the melting point before class time ran out…
If heated from hot water (high temperature):
Example:
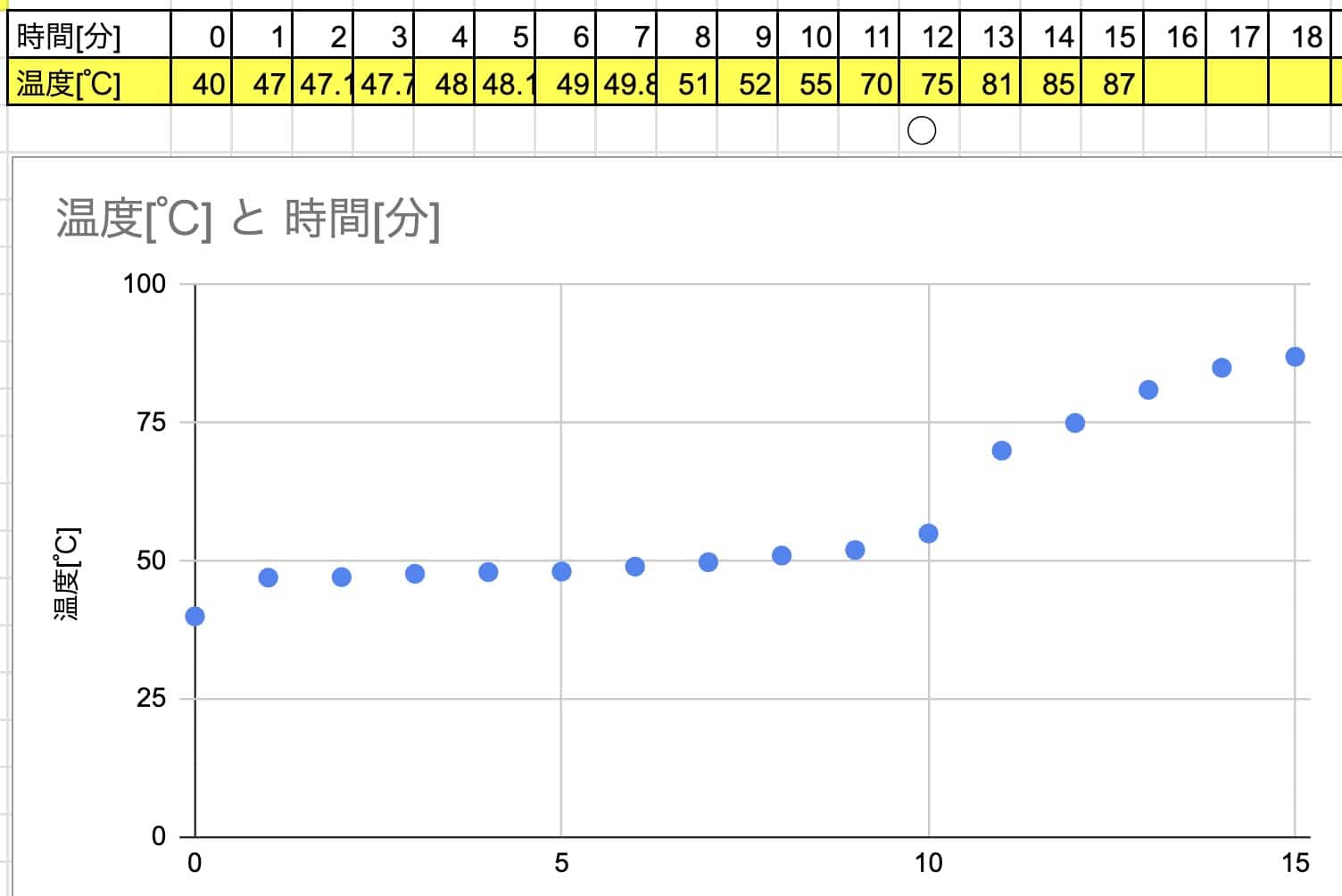
→ Too fast! The temperature change happened in an instant, and we missed the crucial moment where the temperature plateaus…
If heated from lukewarm water (around 30
∘
C):
→ This is the best method! The temperature rises gradually, allowing us to fully observe the drama of the state change!



Why Does the Temperature “Plateau”? The Hidden Mystery
The most intriguing part of the experiment is the “plateau”—that period when you keep heating the substance, but the temperature barely rises while it changes from a solid to a liquid. Why does this stall happen?
The key lies in “Latent Heat.”
As the name suggests, it’s “hidden heat.” In a solid, countless molecules are tightly linked and lined up in a neat, organized structure. When you add energy in the form of heat, the molecules start to vibrate, and the temperature goes up.
However, once the substance hits its melting point, the added heat energy is no longer used to raise the temperature; instead, it’s used to break the bonds connecting the molecules and allow them to move around freely. This is the very essence of a “change of state.”
Until everyone is free and the substance has become a liquid, the heat energy is entirely dedicated to this “bond-breaking work.” That’s why the thermometer reading doesn’t budge. The moment all the bonds are broken and the substance is completely liquid, the heat energy returns to the job of making the molecules move more violently, and the temperature starts to climb again.
The same thing happens when ice turns into water. If you heat ice straight from the freezer, it stays at 0
∘
C (32
∘
F) until it has completely melted, right? That’s the ice molecules using Latent Heat to transform into water.
Conclusion
This experiment was simply about heating palmitic acid, but it unlocked the crucial scientific principle of “Change of State and Latent Heat,” a core concept in middle school science.
The moment the thermometer slows down is proof that, in the unseen world of molecules, heat energy is working hard to change the substance’s state. This is the perfect learning experience where theory and reality perfectly connect.
If you ever have a chance to do a science project at home, remember the motto: “Start with lukewarm water!” Try tracing the temperature change of an everyday substance. You’re sure to be captivated by the fun of science!
Inquiries and Requests
Making the wonder and fun of science more accessible! I summarize easy-to-understand science experiments you can do at home and provide helpful tips. Feel free to search for more!
• Content from Science Notebook is now a book. Learn more here
• About the operator, Ken Kuwako: Click here
• For various requests (writing, lectures, workshops, TV supervision, appearances, etc.): Click here
• Updates on articles are posted on X!
![]() We post experiment videos on the Science Note Channel!
We post experiment videos on the Science Note Channel!
3月のイチオシ実験!
- 押し花を作ろう!:梅や桜の花の押し花を作ってみましょう。特別なケースに入れると、長く保存できて、しおりにもなります。
テレビ番組・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
- 2月27日放送予定「チコちゃんに叱られる」(NHK)の科学監修しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師・ショー・その他お知らせ
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) 秘密兵器「帯電ガン」が炸裂!ビリビリ!ドキドキ!静電気サイエンスショー@千葉市科学フェスタ(午後予定)
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。



