Kitchen CSI: Unmasking the White Suspects (Salt, Sugar, and Flour!)
Every Day is an Experiment.
Take a look at those pure white powders in your kitchen. Sugar, salt, flour. They look identical, but what if they got shuffled up and you couldn’t tell which was which? How would you identify them? (No tasting allowed!) This time, we’re diving into a “Science Mystery” lurking in our daily lives, and we’ll show you an inquiry-based experiment where junior high students rose to the challenge. They were presented with three mysterious white powders: A, B, and C. Could they use the power of science to expose the true identity of these “White Suspects”?
 Here are our “suspects.” Some students might have an educated guess just by looking, but science isn’t satisfied with a “hunch.” We need evidence.
Here are our “suspects.” Some students might have an educated guess just by looking, but science isn’t satisfied with a “hunch.” We need evidence.
The Investigation Begins! What Experiments Will Unmask the Powders?
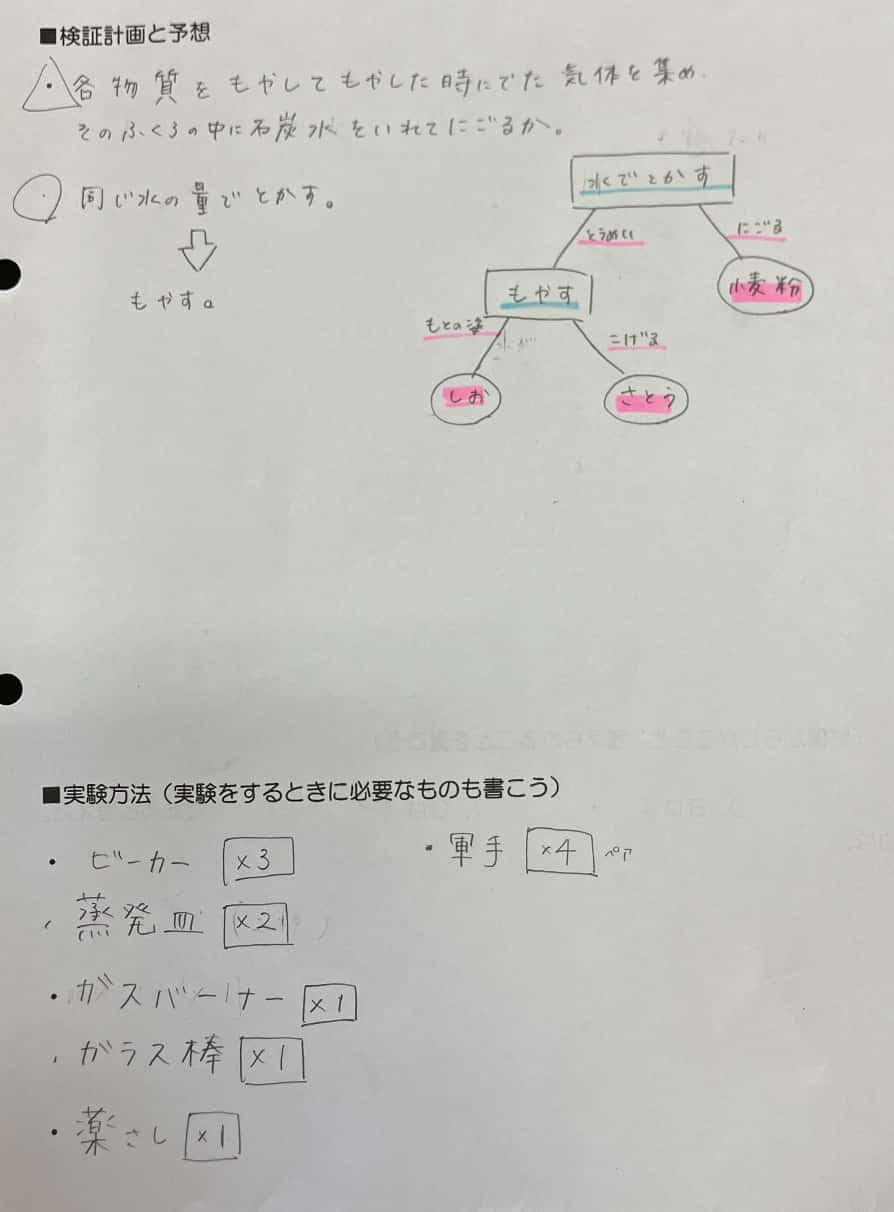
The students were told upfront that the suspects were one of flour, sugar, or salt. The first step was for each group to formulate their “Investigation Plan”—their experimental strategy. As long as it wasn’t dangerous, anything was fair game. That’s the beauty of inquiry.


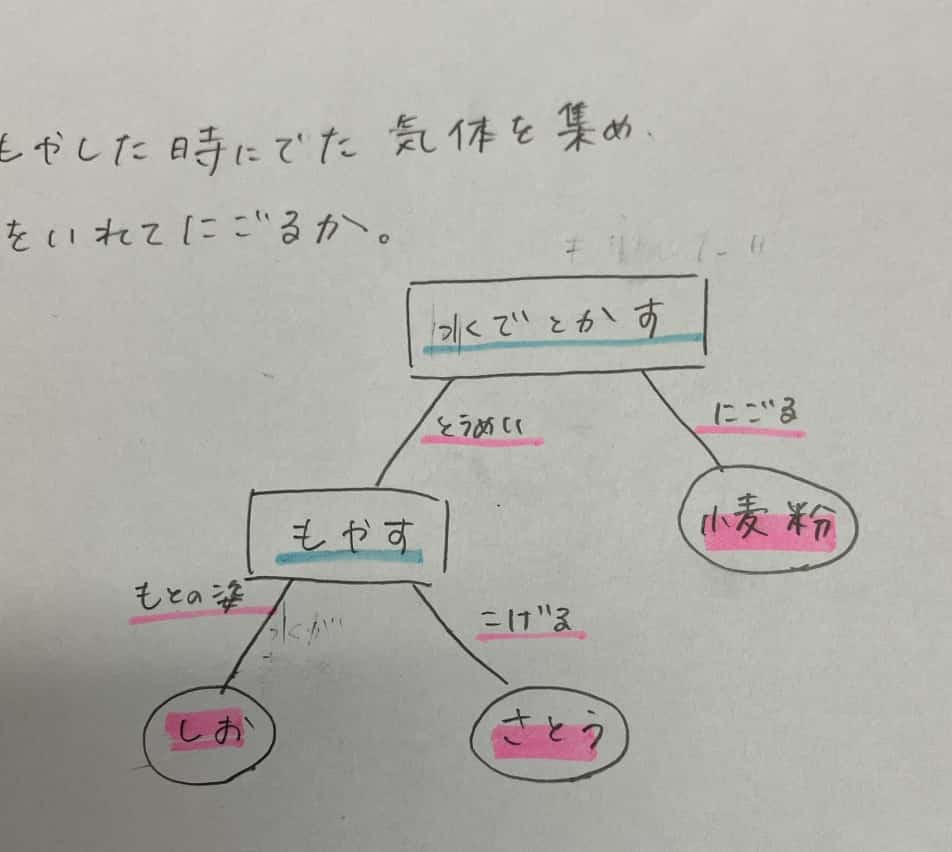
This group’s strategy seems to be dissolving them in water first. And then, burning them…? That’s a pretty bold plan!
Once the plan was set, they wrote down their needed “Investigation Tools”: beakers, test tubes, and… a Bunsen burner. The search for the truth was about to start.
The First Test: Dissolving in Water
The way a substance dissolves in water offers the first major clue.


A clear difference emerged right away! Two of the samples seemed to dissolve into a clear solution, but one turned the water cloudy and settled at the bottom. That’s right—flour. The main component of flour, starch, doesn’t “dissolve” cleanly in water like sugar or salt (it actually swells by absorbing water). With this key finding, the identity of one suspect was all but confirmed!
The Second Test: The Burn to Learn
Next, the students tried “combustion”—literally putting the suspects on trial by fire.


This test provided a definitive contrast! One sample charred black, while the other remained completely white and refused to burn. (Note: salt sometimes makes a “crackling” sound when heated due to trace moisture.)


This difference reveals a fundamental distinction in matter. The ones that charred black were sugar and flour. These are organic compounds, meaning their structure is built on a carbon backbone. When they burn (oxidize), that carbon backbone is left behind as black “charcoal.” On the other hand, the white powder that refused to burn was salt (sodium chloride). Salt is an inorganic compound that contains no carbon. Its inability to burn is a key signature of inorganic matter.
With “didn’t dissolve” (flour) and “didn’t burn” (salt) identified, the remaining suspect that “dissolved and burned” must be sugar!
Case closed… or is it? Other groups were conducting even more thorough “side investigations.”
Conductivity Test


This experiment is electrifying! The students inserted electrodes into the dissolved liquids. Sugar water does not conduct electricity because sugar dissolves as intact “molecules.” However, when they tested salt water… the current flowed! This is because salt (sodium chloride) is an electrolyte; when dissolved, it breaks apart into charged particles (ions)—sodium ions and chloride ions. Even though they look like the same clear liquid, this test exposed a profound difference in their internal makeup.
Back to Powder (Evaporation)

If it dissolved in water, let’s see if we can get it back out! This test involved heating the liquids on an evaporating dish to boil away the water.


The result was crystal clear. From the salt water, the original white salt crystals reappeared. Meanwhile, when the sugar water was heated, the sugar began to change due to the heat (caramelization) and eventually charred black, just like in the burning test. Again, this highlights the crucial difference between organic and inorganic substances.
Litmus Paper Analysis

This group decided to check the acidity/alkalinity using litmus paper. Since the suspects were salt, sugar, and flour, all of them would have been neutral, so there wouldn’t have been a big change. However, what if baking soda (weakly alkaline) or citric acid (acidic) had been among the “suspects”? This litmus paper would have been the key piece of evidence! The ability to adjust the difficulty like this is part of what makes experiments fun.
The Summary of Findings and the Thrill of Science
All investigations (experiments) concluded, and the results were compiled.


“Does it dissolve in water?” “Does it burn?” “Does it conduct electricity?” “What happens when it evaporates?” Based on their own plans, the students logically determined the identities of the white powders—which looked indistinguishable to the naked eye—by testing their various “properties.” For instance: “A didn’t burn and conducted electricity, so it’s salt.” “B didn’t dissolve in water and charred, so it’s flour.” “C dissolved in water and charred, so it’s sugar.”
This is the excitement of science. It’s not just about memorizing facts; it’s about using knowledge to pose a question, test a hypothesis, and derive the truth. We barely finished clean-up before the 50-minute class was over, but we took care of the rest!

Your own kitchen is full of “science seeds” waiting to be discovered. Why not play the role of a science detective and take a fresh look at the world around you?
Inquiries and Requests
Let’s make the wonders and excitement of science more accessible! We’ve gathered easy-to-understand tips and fun science experiments you can do at home. Feel free to search around!
The content of our science blog has been turned into a book. Find out more here.
Learn more about the administrator, Ken Kuwako, here.
For various requests (writing, lectures, experiment classes, TV supervision, appearances, etc.), click here. * Updates are posted on X!
![]() We post experiment videos on the Kuwako Science Channel!
We post experiment videos on the Kuwako Science Channel!


