The Ultimate Battery Battle: Which Two Metals Create the Most Power? (The Secret is a Metal’s “Personality”)
I’m Ken Kuwako, Science Trainer. Every Day is an Experiment.
Smartphones, game consoles, TV remotes… our lives are impossible without batteries, right? But have you ever stopped to think about how batteries actually generate electricity? The truth is, anyone can easily make a battery with just two different types of metal and “a certain liquid.”
This time, we’re introducing an exciting experiment to find out which combination of metals makes the “Ultimate Battery.” And the main ingredients? Hydrochloric acid, combined with metals you already know!
The Experiment: Battle for the Strongest Battery!
First, let’s introduce the “contestants” in this experiment—the following four electrode materials:
- Copper Plate
- Zinc Plate
- Aluminum Plate
- Carbon Rod
How can we combine these to create the most powerful (highest voltage) battery? We’re going to try it out! The base we use to hold the electrodes? None other than a Styrofoam board! It’s perfect because it doesn’t conduct electricity, keeping the electrodes separated and securely in place.



Next, we immerse the board, with the electrodes set, into the liquid that serves as the “stage” for our contestants. That liquid is… Hydrochloric Acid (3–5% concentration, 40mL)!

Hearing “Hydrochloric Acid” might be a bit alarming, but the low concentration we’re using makes it relatively safe. Nevertheless, since it is an acid, if it gets on your hands, be absolutely sure to wash them immediately and thoroughly with water.
The Question: Which Combination is the Strongest?
Now, for a quick quiz! Out of Copper, Zinc, Aluminum, and Carbon, which two do you think will produce the highest voltage when combined?
“The classic ‘Copper and Zinc’ from the textbooks looks promising!” “Maybe the lightweight aluminum will surprise us?” “Carbon isn’t even a metal, how will it do?”
Let’s form some hypotheses and dive into the experiment! That moment of thinking, “I wonder what will happen?” is the most exciting part of science.
The Shocking Results and the “Mechanism” of a Battery
A tip: The carbon rod can be tricky to attach alligator clips to, but if you slightly loosen the screw portion and clip it there, it works perfectly.

Here’s a look at measuring one of the combinations:
And here are the results after measuring the voltage for various patterns!
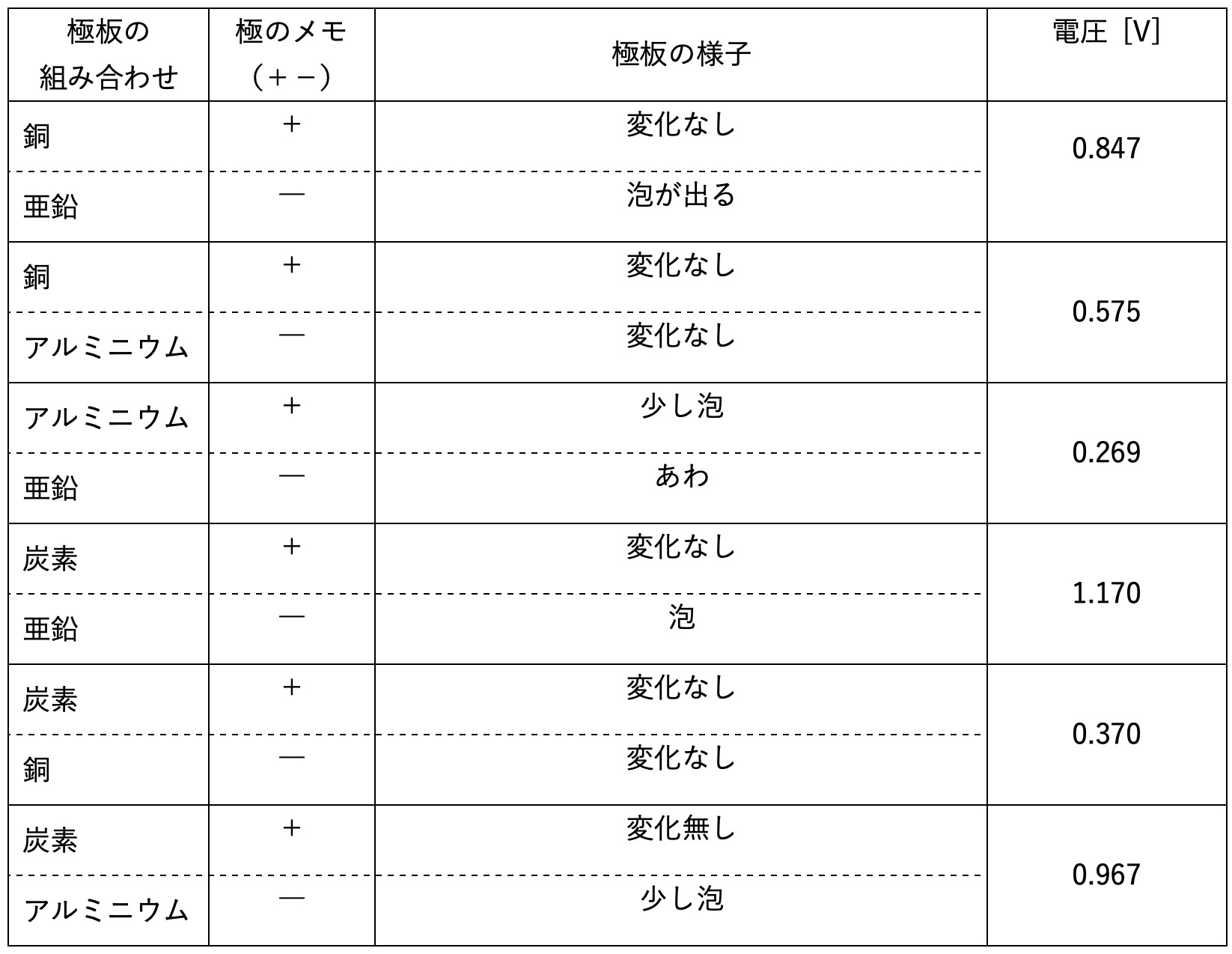
The classic combination of “Copper and Zinc,” famous from science classes, produced about 0.84V. This is actually the blueprint for the world’s first practical battery, the “Voltaic Pile,” invented by the Italian scientist Alessandro Volta around 1800.

The Secret: A Metal’s “Personality” Determines the Voltage!
So, why does the voltage change depending on the combination? The answer is closely tied to a property of metals that we can think of as their “personality”: Ionization Tendency (or Electromotive Force Series). Simply put, ionization tendency is the degree to which a metal “wants to dissolve (in water or acid) and become an ‘ion.'” When a metal becomes an ion, it releases electrons (particles of electricity).
Looking at our contestants:
- Aluminum, Zinc: The “I really want to become an ion and aggressively release electrons!” type (Has a High ionization tendency).
- Copper, Carbon: The “I’d rather not become an ion…” type (Has a Low ionization tendency).
A battery works by using the difference in this “desire to become an ion” to force electrons to flow from one material to the other (generating an electric current). In short, the greater the difference, the stronger the force pushing the electrons (the voltage)!
That’s why combining Aluminum—which strongly wants to be an ion—with Carbon—which barely wants to—created the highest voltage! The scientific story is now clear!
Try it Yourself! Compare Your Results!
We’ve prepared a spreadsheet where you can record your experiment results. If you try this at school or home, be sure to compare your findings with others!
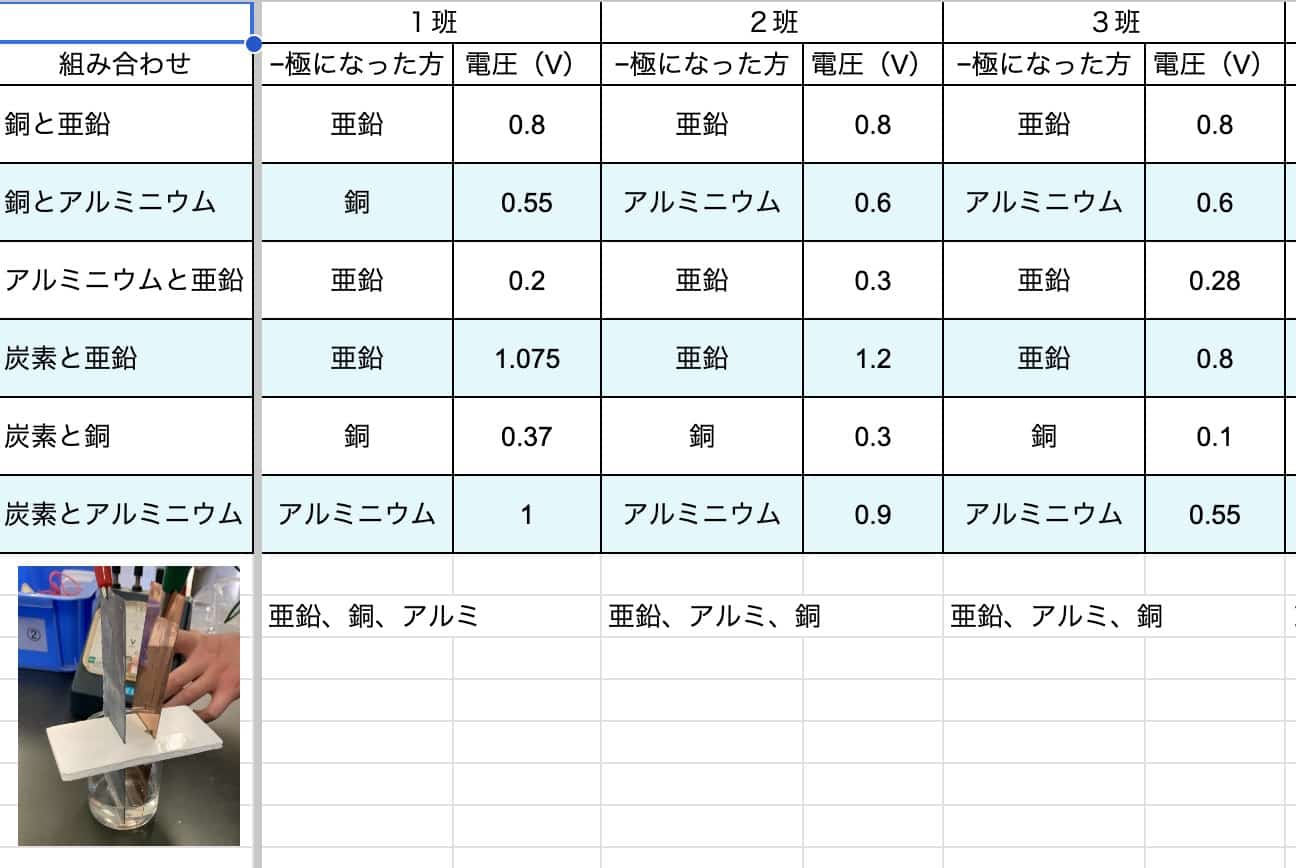
Spreadsheet for Creating Various Batteries
This experiment is not only easy to do with simple materials but also powerfully stimulates your curiosity: “Why did that happen?” By the way, the historical “Voltaic Pile” we mentioned earlier had a major weakness (polarization)—its voltage would quickly drop. Science has evolved by overcoming such flaws! If you’re interested, please check out this related article: “Stop Using the Voltaic Pile.” Even the “obvious” things in our daily lives are full of new discoveries when viewed through the lens of science!
Contact and Requests
Bring the wonder and fun of science closer to you! This site provides easy-to-understand summaries of fun science experiments you can do at home, along with tips and tricks. Feel free to search around! ・The content of this science blog is now available as a book. Find out more here ・Find out more about the site operator, Ken Kuwako, here ・For various requests (writing, lectures, science classes, TV supervision/appearances, etc.), please contact us here ・Article updates are posted on X (formerly Twitter)!
![]() We post experiment videos on the Science Idea Channel!
We post experiment videos on the Science Idea Channel!


