[No More Confusion!] The Ultimate Chemistry Recipe to Convert Mass Percent, Molarity, Normality (N), and ppm with Ease
This is Ken Kuwako, the Science Trainer. Every day is an experiment.
Have you ever been following a recipe and wondered what “a pinch of salt” really means? In the world of chemistry, we have a similar situation with several ways to express “concentration,” and scientists choose the best one for the job. Today, let’s explore how to switch between two of these units: mass percent concentration, which you might remember from science class, and the more professional molar concentration (molarity). Understanding these calculations will make the world of chemistry feel much more approachable, and your experiments will become more fun—and safer!
For details on how to actually dilute solutions, please see this article.
The Basics of Concentration: Understanding the Difference in Units
In junior high textbooks, “mass percent concentration” is common, but in professional lab manuals and research papers, “molar concentration” is the standard. So, let’s master how to convert between these two freely. Knowing the calculation method is not only convenient, but most importantly, it gives you confidence.
Both mass percent and molar concentration share the same basic idea:
Solute (what’s dissolved) ÷ Solution (the whole thing)
The only difference is the units. Mass percent concentration is based on mass (g).
Solute (g) ÷ Solution (g)
Molar concentration, on the other hand, uses the mole (mol), which focuses on the number of particles for chemical reactions, and volume (L).
Solute (mol) ÷ Solution (L)
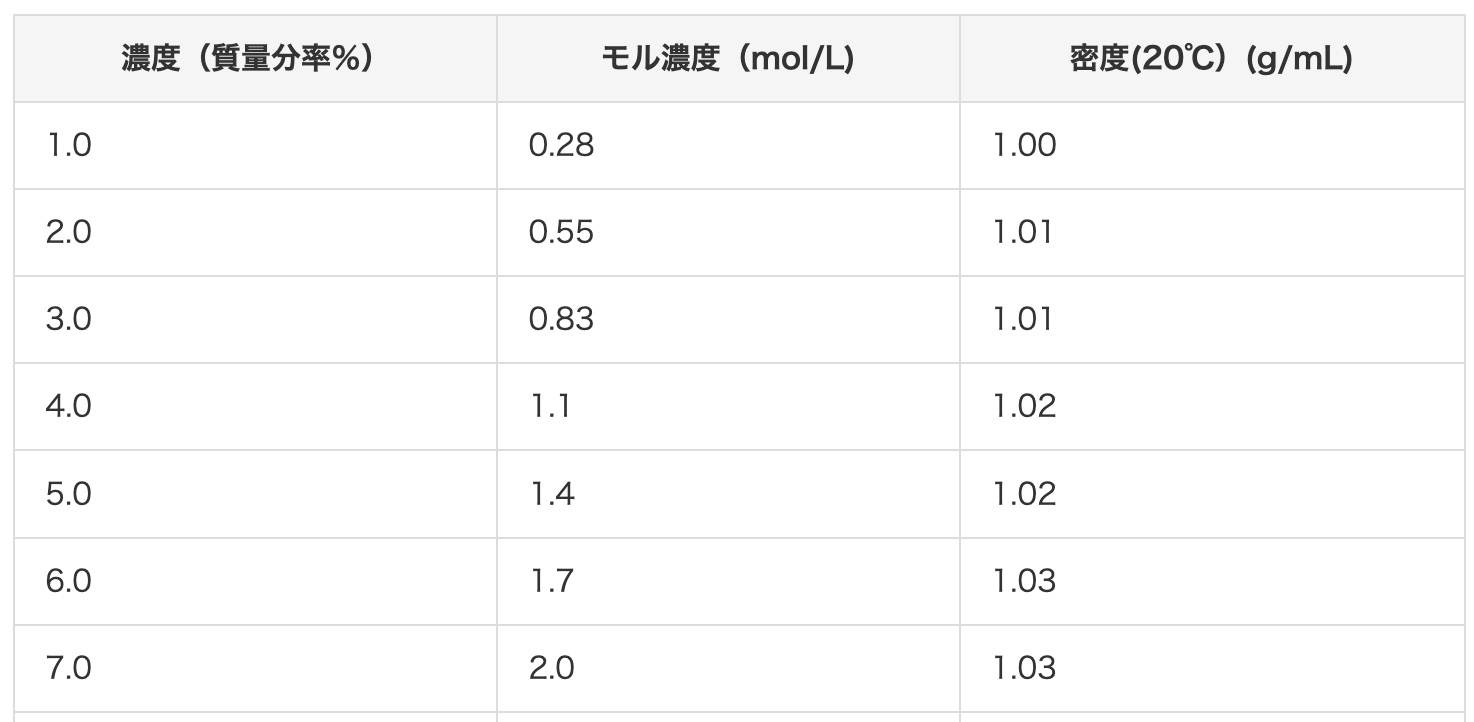
Let’s try a real example and convert a 2 mol/L hydrochloric acid solution to mass percent concentration. According to this conversion chart, 2 mol/L hydrochloric acid should be about 7%. Let’s do the math and see if that’s true!
Practice! From Molar Concentration to Mass Percent
First, let’s gather the necessary information. The molecular weight of hydrochloric acid (HCl): We add the atomic weights. Hydrogen (H) is 1 and Chlorine (Cl) is 35.5, so 1 + 35.5 = 36.5. The density of 2 mol/L hydrochloric acid: This source says it’s 1.03 g/cm³. This means every 1 cm³ has a mass of 1.03 g.
Now, let’s base our calculation on 1 L of the solution.
Solute: 2 mol ⟶ 2 × 36.5 = 73 g
Solution: 1 L = 1000 cm³ ⟶ 1000 × 1.03 g/cm³ = 1030 g
So, we have 73 g of hydrogen chloride dissolved in 1 L (1030 g) of the solution. If we plug this into the mass percent formula…
73 (g) ÷ 1030 (g) = 0.070… In other words, about 7%!
It perfectly matches the value from the chart!
Let’s Try the Reverse! From Mass Percent to Molar Concentration
Next, let’s tackle the reverse calculation. As long as we convert the units one by one, we can always get back to where we started. For example, let’s find the molar concentration of a 7% hydrochloric acid solution with a density of 1.03 g/mL. The key to molarity is figuring out “how many moles of solute are in 1 L of solution.”
First, let’s calculate the mass of 1 L of the solution.
Solution: 1 L = 1000 mL ⟶ 1000 mL × 1.03 g/mL = 1030 g
Of this, 7% is the mass of the solute (hydrogen chloride).
Solute: 1030 g × 0.07 = 72.1 g
Finally, let’s convert the mass of the solute into moles. We divide by the molecular weight of hydrogen chloride (36.5).
72.1 (g) ÷ 36.5 (g/mol) ≒ 1.97 (mol)
Since there are 1.97 moles in 1 L of the solution, the concentration is about 2 mol/L. Nailed it again!
Helpful Calculation Tools
It seems someone has created a concentration conversion tool for commonly used chemicals like hydrochloric acid and sodium hydroxide. It’s a great way to double-check your work.
https://keisan.casio.jp/menu/person/TaikiIkekame
For instance, with hydrochloric acid, you can use this site and just enter “7%” to immediately get a precise value of “1.981 mol/L.”
https://keisan.casio.jp/exec/user/1480248881

[Fun Fact #1] What’s the Difference Between Hydrochloric Acid and Hydrogen Chloride?
People often mix these up, but hydrogen chloride (HCl) is a gas at room temperature. When that gas dissolves in water, the resulting aqueous solution is called hydrochloric acid. In fact, the stomach acid in our bodies is mainly hydrochloric acid, and it helps us digest food! Just be careful not to mistake “hydrogen chloride (HCl)” for just “chlorine (Cl).”
[Fun Fact #2] What is Normality (N)?
Another unit called Normality (N) also exists. It’s the molar concentration multiplied by the “power number” (valence) of an acid or base. For example, hydrochloric acid (HCl) has a power of 1 (it’s monovalent), so if its molarity is 1 mol/L, its normality is also 1 N. On the other hand, sulfuric acid (H₂SO₄) has a power of 2 (divalent), so 1 mol/L would be 2 N. It’s a handy unit when you want to intuitively compare the reactive strength of acids and bases. For more details, please see here.
[Fun Fact #3] How small is ppm?
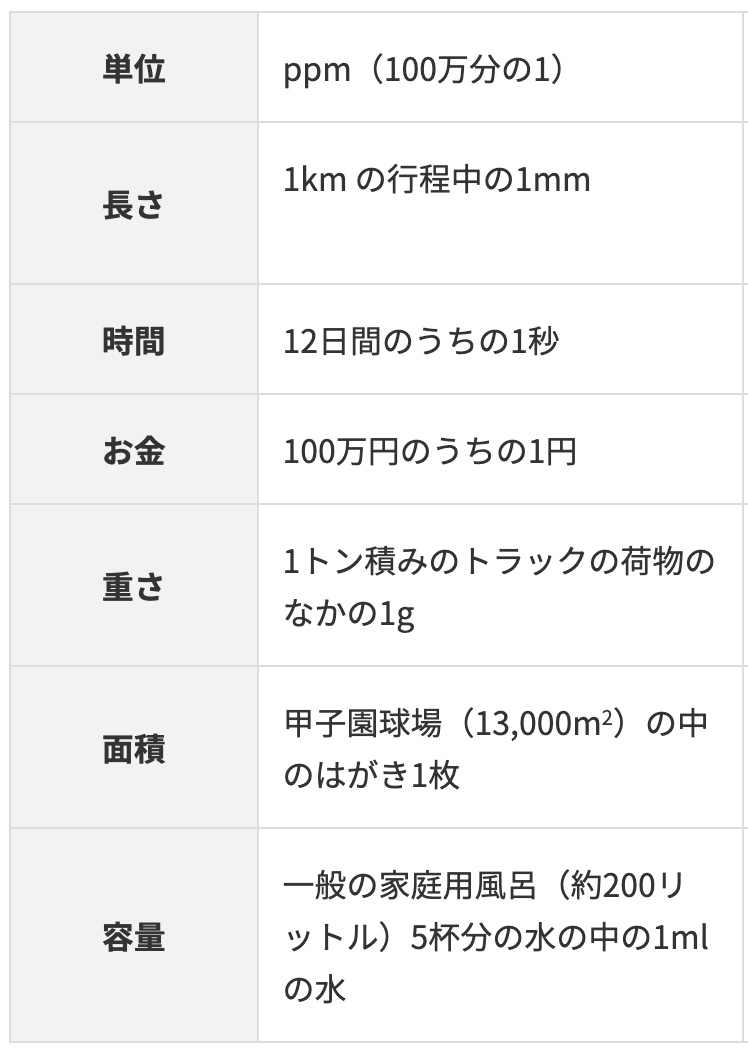
“ppm” stands for “parts per million” and means “1 in a million.” While percent is parts per hundred, ppm is parts per million. It’s used to describe extremely small concentrations.
Mass of solute (mg) / Mass of solution (kg)
A state of 1 ppm is when just 1 mg of a solute is dissolved in 1 kg (1000 g) of solution.
Just how small is that?
- Just 1 gram within a 1-ton truck.
- 1 yen out of 1 million yen.
- I heard at a workshop that the odds of winning the grand prize in the New Year’s postcard lottery in Japan is about 1 ppm! (I looked it up).
Thinking about it this way, you can really feel how incredibly tiny a ppm is.
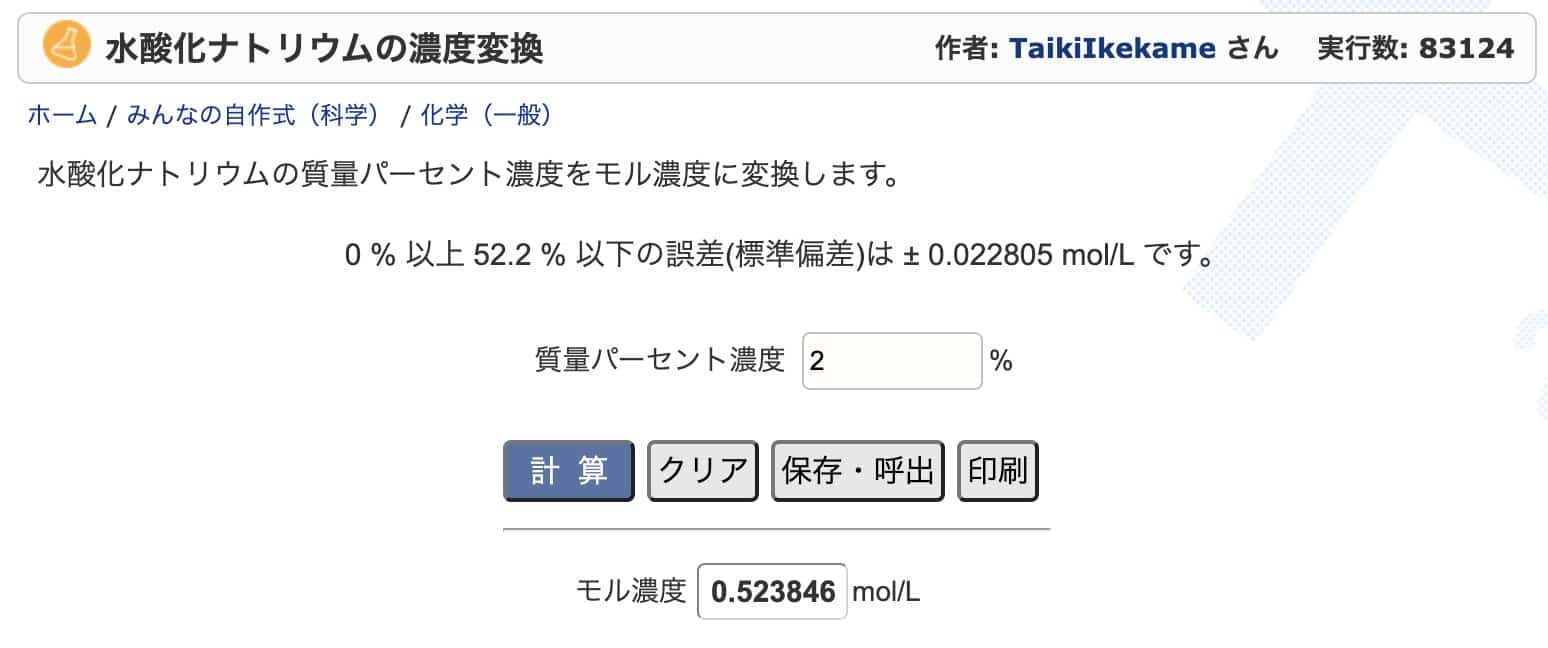
Practice Problem: Let’s Try Converting Sodium Hydroxide
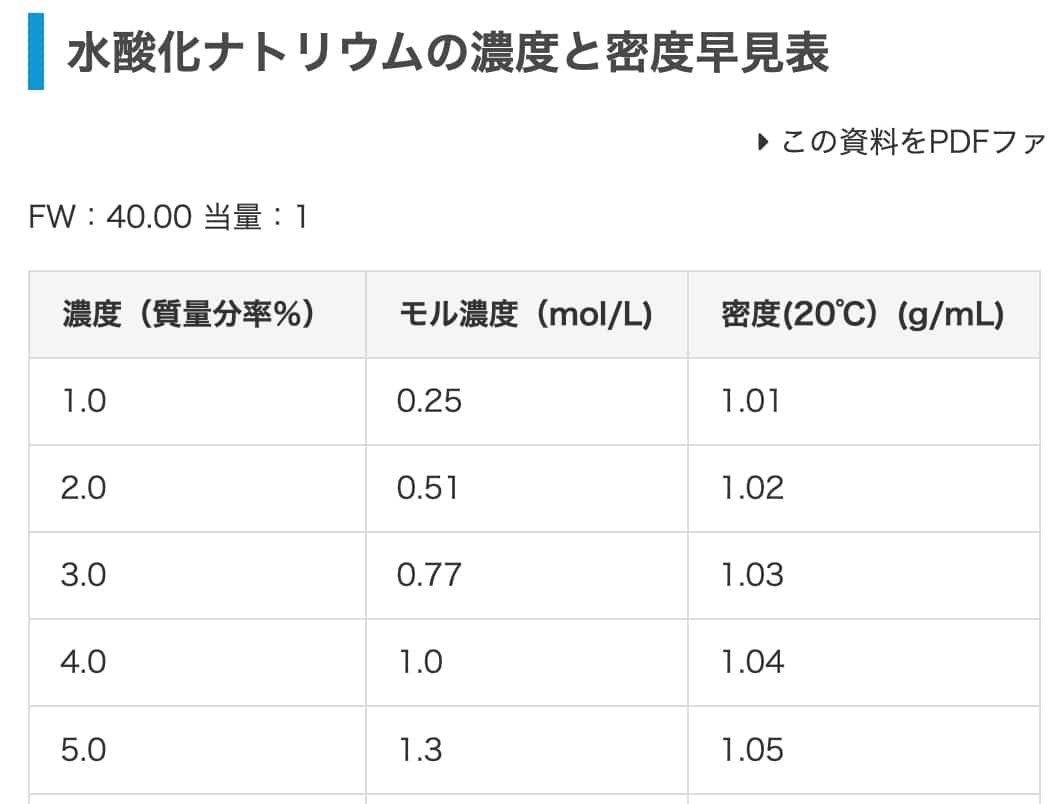
Finally, let’s practice with sodium hydroxide. We’ll convert a 2% sodium hydroxide solution to molar concentration. Referring to this chart, the density is 1.02 g/mL (it seems the 1.03 g/mL in the original article was for HCl, so we’ll use this value). The answer should be 0.51 mol/L.
First, calculate the mass of 1 L of the solution.
1000 mL × 1.02 g/mL = 1020 g
2% of this is the amount of solute.
1020 g × 0.02 = 20.4 g
Now, convert this to moles. The molecular weight of sodium hydroxide (NaOH) is 40.
20.4 g / 40 (g/mol) = 0.51 mol
So, with 0.51 moles per liter, the molar concentration is 0.51 mol/L. It’s a perfect match with the chart’s value!
Even if you check it on this website, the results are nearly identical.
Sodium Hydroxide Concentration Conversion: https://keisan.casio.jp/exec/user/1480256880
Reference
For the hydrochloric acid often used in middle school, it’s convenient to dilute concentrated HCl all at once. For example, to make a 10% HCl solution, you add 3.2 parts water to 1 part concentrated HCl by volume. To turn a whole 500 cm³ bottle of concentrated HCl into a 10% solution, you would dilute it with 1600 cm³ of water. It’s useful to dilute and store it in a 3-liter polyethylene container (Amazon).
|
|
Contact & Inquiries
Bringing the wonder and fun of science closer to you! I create easy-to-understand guides for fun science experiments you can do at home. Please feel free to search for various topics!
・About the operator, Ken Kuwako, click here.
・For various requests (writing, lectures, experiment workshops, TV supervision, appearances, etc.), click here.
・Article updates are posted on X (formerly Twitter)!
![]() Check out experiment videos on my Science Content Channel!
Check out experiment videos on my Science Content Channel!
2月のイチオシ実験!梱包材で遊ぼう!
- 静電気の時期になってきました。子供と一緒に梱包材で盛り上がろう!→ やめられなくなる!静電気実験20
 体中に梱包材をはりつけてみよう!
体中に梱包材をはりつけてみよう!
テレビ番組等・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師等・ショー・その他お知らせ
- 2/20(金)「生徒の進学希望実現支援事業」研究授業@福井県立若狭高等学校 講師
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) サイエンスショー予定
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。

![[商品価格に関しましては、リンクが作成された時点と現時点で情報が変更されている場合がございます。] [商品価格に関しましては、リンクが作成された時点と現時点で情報が変更されている場合がございます。]](https://hbb.afl.rakuten.co.jp/hgb/2c6a2576.25faaa1b.2c6a2577.0abda625/?me_id=1240371&item_id=10000489&pc=https%3A%2F%2Fthumbnail.image.rakuten.co.jp%2F%400_mall%2Fnagamineshouten%2Fcabinet%2Fporikan%2F3-1.jpg%3F_ex%3D80x80&s=80x80&t=picttext)