Squish the Air! A Visual Experiment to Reveal Boyle’s Law
Ken Kuwako, Science Trainer. Every day is an experiment.
Air is invisible, yet it’s always pushing us with surprising strength. What do you think would happen if you trapped that air and squeezed it even tighter? Today, we’re going to dive into the mysterious relationship between air’s “force (pressure)” and its “size (volume)” with an experiment exploring Boyle’s Law. You might just uncover the secret behind why a bag of potato chips puffs up on a mountaintop!
The Tools We Used
For this experiment, we used a science kit from Narika. The kit included a large syringe, a pressure gauge, and a rubber tube to connect them. It’s a simple setup, but it’s enough to unlock the secrets of air.
Experiment Starts! Compressing the Air
First, the setup. We attached the rubber tube to the syringe and connected the pressure gauge. We started at the 50ml mark so it would be easy to read the volume of air inside the syringe.

The manual also specified 50ml, so we’re keeping the conditions the same.
At this starting point, the pressure gauge reads about 100kPa (kilopascals). This is roughly the same value as the atmospheric pressure (the pressure caused by the weight of the air) in our daily environment.
From here, we started pushing the syringe piston firmly. As we decreased the volume of the trapped air… just as expected, the pressure gauge reading shot right up!

The Results Are In! The Beautiful Relationship Between Pressure and Volume
We measured the volume of air at different points by adjusting the force we applied to the piston: when the pressure was 100kPa (the start), 110kPa, 120kPa, and so on. Here is the compiled data:
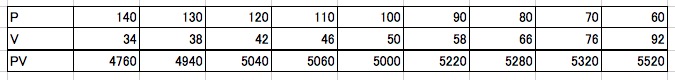
These numbers might not tell the full story on their own, but when we plot them on a graph (called a P-V graph), an astonishingly beautiful relationship emerges.
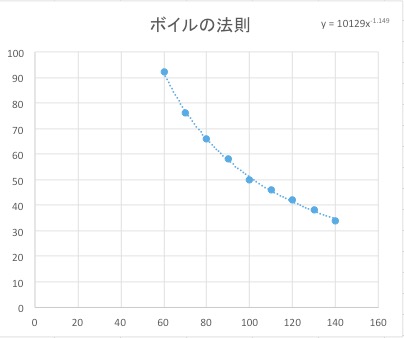
Take a look! This curve is exactly what you see in a math class when studying “inverse proportion”! In other words, the relationship hidden here is that “when you halve the volume, the pressure doubles.”
Why is it an Inverse Proportion?
So, why does this relationship occur? Although air is invisible, it is made up of countless “tiny particles of air (molecules)” zipping around. Pressure is created when these particles collide with the walls of the syringe.
What happens when you push the syringe and narrow the volume (the room)? The space where the particles can move around is reduced, causing them to collide with the walls more frequently. This is what we mean by “the pressure goes up.” If you cut the volume in half, the frequency of collisions simply doubles. That’s why pressure and volume have a clean, inversely proportional relationship. This, in a nutshell, is Boyle’s Law.
A Challenge in the Classroom
I bought one of these experiment kits and immediately had my students try it out in class. We had representative students push and pull the piston while everyone else read the measurements, recorded the data, and plotted the points on graph paper.
Instead of just learning from a textbook that “it’s an inverse proportion,” the students were able to truly grasp the properties of air through the experience of “seeing it with their eyes, feeling it with their hands, and drawing the graph.” Since the law can be confirmed so easily, I’m hoping to get one for every group!

Contact and Requests
Bring the wonder and fun of science closer to home! I’ve put together easy-to-understand tips and fun science experiments you can do at home. Feel free to browse around! ・The content from the Science Notebook is now a book. Find out more here ・About the manager, Ken Kuwako: click here ・For various requests (writing, lectures, workshops, TV supervision, appearances, etc.): click here ・Updates on articles are posted on X!
![]() Experimental videos are available on the Science Idea Channel!
Experimental videos are available on the Science Idea Channel!
2月のイチオシ実験!梱包材で遊ぼう!
- 静電気の時期になってきました。子供と一緒に梱包材で盛り上がろう!→ やめられなくなる!静電気実験20
 体中に梱包材をはりつけてみよう!
体中に梱包材をはりつけてみよう!
テレビ番組等・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師等・ショー・その他お知らせ
- 2/20(金)「生徒の進学希望実現支援事業」研究授業@福井県立若狭高等学校 講師
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) サイエンスショー予定
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。


