Try Making Your Own Fireworks! A Simple Chemistry Experiment (Black Powder & Dry Distillation)
Make Your Own Fireworks and Brighten Up Summer Nights!
Why not make a summer night even more special with homemade fireworks? Let’s try making your own sparklers! Perfect for warm evenings, sparklers can actually be made at home. You’ll need just a few materials: wooden chopsticks, sulfur, potassium nitrate, and Japanese paper (washi). With these, you can create your own original sparkler using homemade black powder.
Here’s a simple overview of the process:
-
Carbonize the chopsticks: This “dry distillation” involves heating the chopsticks at high temperature until they turn into charcoal.
-
Make black powder: Mix the charcoal with sulfur and potassium nitrate to create homemade black powder.
-
Wrap the powder in paper: Use the washi paper to carefully wrap the powder—this is where a bit of craftsmanship comes into play.
Now for the fun part: lighting it! If done correctly, you’ll see small, round sparks, sparkling as they burn—just like store-bought sparklers. It’s almost like launching your very own miniature fireworks into the summer night sky.
Safety first: Because you’re handling explosive materials, always do this activity with an adult and follow proper safety precautions.
Detailed Experiment Procedure
Materials: Wooden chopsticks, potassium nitrate, sulfur, iron powder
Equipment: Gas burner, test tube, rubber stopper with glass tube, mortar and pestle, washi paper, water tank
Note: If you prefer not to make charcoal from chopsticks, you can use commercially available activated charcoal powder instead. This is a convenient alternative for beginners.
Making Charcoal from Chopsticks
First, we steam-bake (dry distill) the chopsticks, heating them while cutting off exposure to air, to separate the volatile and non-volatile components. The chopsticks break down into charcoal, wood gas (flammable gas), wood tar (a dark, sticky liquid), and wood vinegar (a yellow liquid).
Pack the chopsticks into a test tube. Keep in mind that the test tube may get ruined, so it’s best to use an old or slightly dirty one.

Important safety notes:
-
Always set the test tube mouth down, or it could break.
-
Never seal the test tube completely.
Dry distillation is a topic sometimes seen in middle school exams. Once you start heating, white smoke will begin to appear. It’s fun to try lighting this gas carefully—here’s a video showing it in action.

The process is complete when no more gas comes out. Discard the wood vinegar, and take out the charcoal. This should yield about 0.6 grams of charcoal.

Making Black Powder
Next, mix 0.4 g of charcoal, 0.6 g of sulfur, and 3.0 g of potassium nitrate in a mortar until well combined. If you’re not making charcoal yourself, you can use commercially available powdered charcoal.
This mixture is black powder, historically used in firearms such as matchlock guns. Each component has a role:

-
Charcoal: acts as the fuel.
-
Sulfur: lowers the ignition point (232°C), since charcoal alone ignites at around 250–300°C.
-
Potassium nitrate: serves as an oxidizer, supplying oxygen for combustion.
You will get a gray powder.
Take about two small spoonfuls of this black powder and place it on a strip of thin Japanese paper (washi). Then carefully twist the paper by hand.

Here’s an important point: the way you place the powder affects how well the sparkler will burn.
-
Cut the paper into strips about 2.5 cm wide.


-
Place the black powder along the strip, adding slightly more in the middle. (In the diagram, I highlighted it with a marker for clarity.)
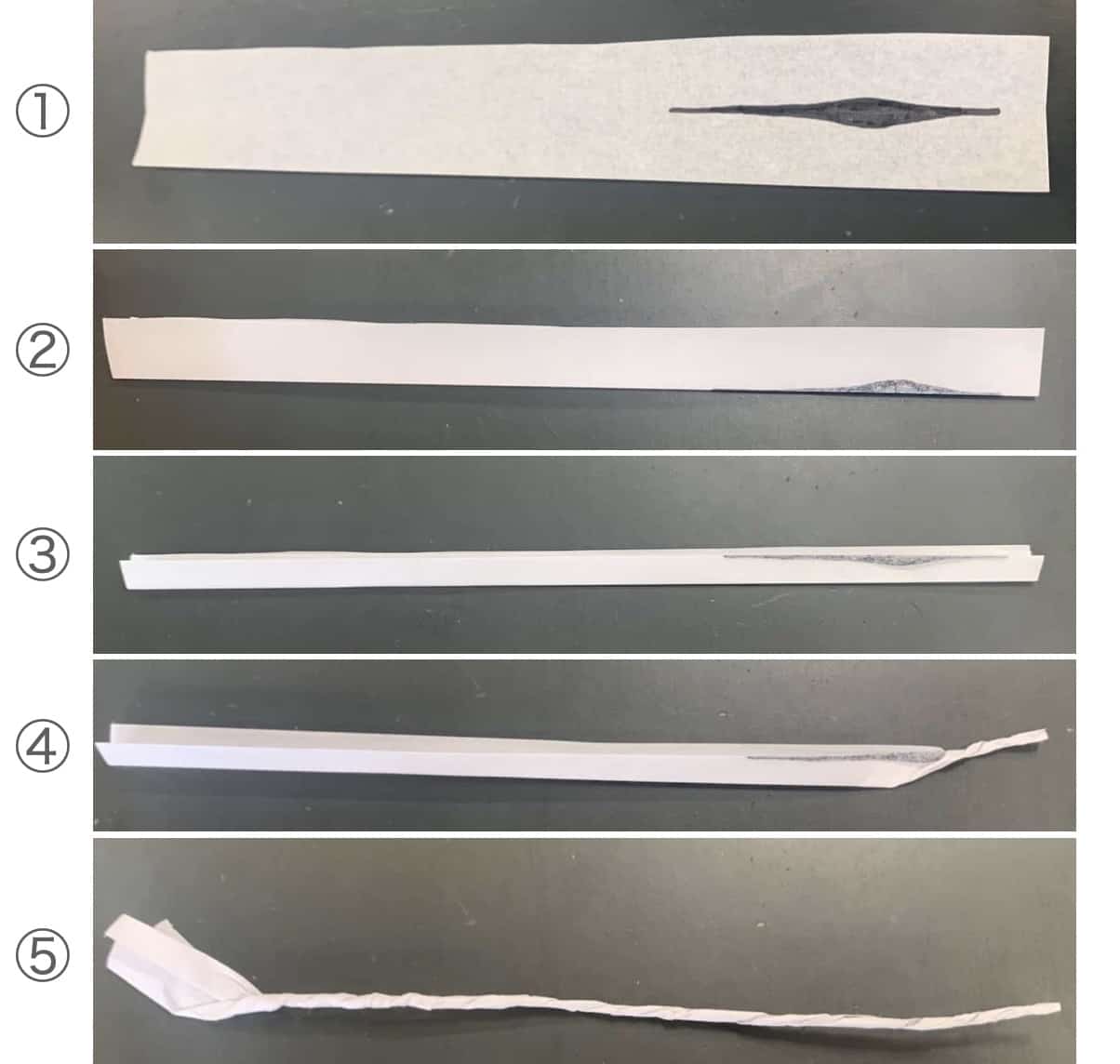


-
Fold the paper carefully to remove air, as shown in steps 2 and 3.


-
Finally, twist the paper tightly as in steps 4 and 5—then your sparkler is complete.
Use a small medicine spoon to scoop the powder onto the paper, and twist it carefully by hand.
Safety tips:


-
Always work over a water-filled container.
-
Hold the top with test tube tongs while burning the bottom end.
-
If it feels too hot, release it immediately.
If done correctly, you’ll see a small, bright spark, just like a store-bought sparkler.
Here’s a video showing a successful sparkler in action.
2月のイチオシ実験!梱包材で遊ぼう!
- 静電気の時期になってきました。子供と一緒に梱包材で盛り上がろう!→ やめられなくなる!静電気実験20
 体中に梱包材をはりつけてみよう!
体中に梱包材をはりつけてみよう!
テレビ番組等・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師等・ショー・その他お知らせ
- 2/20(金)「生徒の進学希望実現支援事業」研究授業@福井県立若狭高等学校 講師
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) サイエンスショー予定
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。






