Why Does Salt Water Conduct Electricity but Sugar Water Doesn’t? The Shocking Secret of Kitchen Chemistry!
I’m Ken Kuwako, your Science Trainer. Every Day is an Experiment!
When you hear the phrase “something that conducts electricity,” you might immediately picture shiny metals. But did you know that you can create a mysterious liquid that carries an electric current just by dissolving a common ‘powder’ found right in your kitchen?
The science experiment we’re introducing today is full of wonder and discovery. We’re diving into the profound yet simple mystery: “Why does salt water conduct electricity, but sugar water doesn’t?” The substances we’ll use—salt, sugar, and ethanol—are all things you’ve heard of. Through this experiment, we’ll unlock the electrical secrets hidden in water. Prep time is about 40 minutes. Ready to open the door to the world of science?
Introducing Our Experimental Lineup! Who are they?
Here are the aqueous solutions and solids starring in this experiment. Some of the clear liquids look exactly alike, but they behave completely differently when faced with electricity.
Purified Water (H₂O)
Salt Water (NaCl aq)
Sugar Water (Sucrose Solution C₁₂H₂₂O₁₁ aq)
Hydrochloric Acid (HCl aq)
Ethanol Solution (C₂H₆O aq)
Sodium Hydroxide Solution (NaOH aq)
Solid Salt (NaCl)
Solid Sugar (C₁₂H₂₂O₁₁)
We’ll be checking which of these “conducts current” and which “does not.” The key to this difference is the existence of tiny particles called ions. Substances that dissociate (split) into positively and negatively charged ions when dissolved in water are called electrolytes. These ions act as “electric carriers,” moving freely through the solution to allow current to flow.
In contrast, substances that dissolve in water but remain as molecules without splitting into ions are called non-electrolytes. Since they lack these electric carriers, no current flows. So, which one is the electrolyte: salt or sugar?

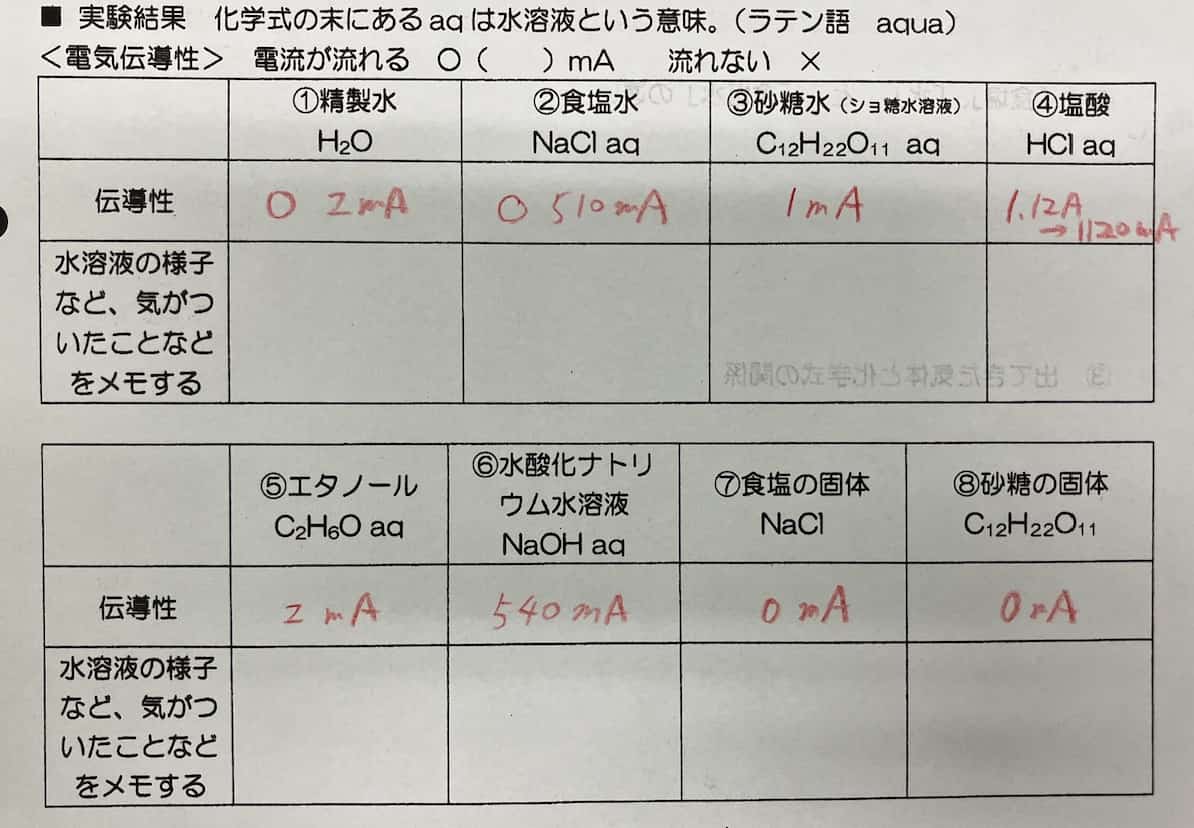
Solutions 1-4





※ Note: Salt water and hydrochloric acid must be replaced for each class because they change color due to a reaction with the electrodes during the experiment. This color change is, in fact, evidence of an exciting chemical reaction.
Time to Experiment! Tracing the Path of Electricity
The students, working like careful scientists, transfer the solutions into cell plates and immerse the stainless steel electrodes. After setting the voltage to 5V and flipping the switch… will the ammeter needle move, or will it remain silent? Everyone watches with bated breath.


After measuring each solution, it’s crucial to thoroughly rinse the electrodes with distilled water. If other solutions mix in, the results won’t be accurate. It’s painstaking work, but it’s the first step toward precise data.
What is the identity of the bubbles generated by Hydrochloric Acid?
The experiment with hydrochloric acid (HCl aq) brought a particularly dramatic phenomenon. The moment the electrodes were immersed, a large current of 1.13A flowed, and a vigorous stream of bubbles erupted from one of the electrodes!

These bubbles are a product of electrolysis, the process where electricity works its magic on a solution. The ions in the hydrochloric acid, energized by the electricity, transformed into different substances: hydrogen gas (H2) and chlorine gas (Cl2). After a while, a distinct, pool-like chlorine scent began to fill the air, and the solution turned a faint yellow.

Before and After the Experiment

The Mystery of the Color Change in Salt Water
An interesting change was also observed in the salt water (NaCl aq) experiment. When current flowed, the solution gradually turned a cloudy orange. What could be the cause of this?

Before and After the Experiment
Salt water after electrolysis. An orange precipitate forms.

In fact, the salt water itself didn’t change color; rather, iron ions dissolved out of the stainless steel anode (+ electrode), forming an orange precipitate called iron(III) hydroxide. The fact that the “stage props”—the electrodes—are also involved in the reaction reveals the complexity of chemistry.
Leaving the current on for longer makes the color clearly visible. Left is hydrochloric acid, right is salt water.

For a more detailed explanation of why this happens, please check the links below.
In the case of Hydrochloric Acid → Chlorine gas is generated at the anode and dissolves in water, changing the color to yellowish-green.
In the case of Salt Water → The stainless steel electrode reacts, and iron(III) hydroxide is released from the anode, causing a brownish-yellow cloudiness.
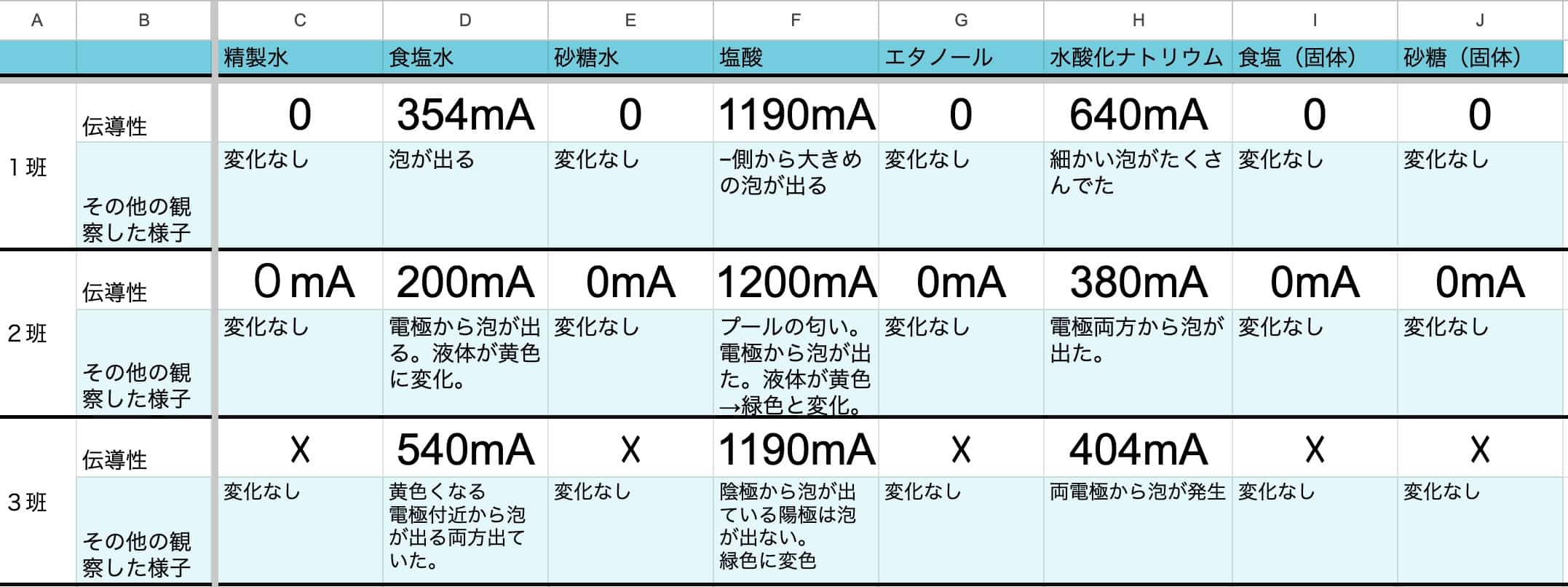
The Final Results! Which Ones Conducted Current?
Let’s summarize the results after all the experiments are complete.
[Conductors (Electrolytes)] Salt Water, Hydrochloric Acid, Sodium Hydroxide Solution
[Poor Conductors (Non-electrolytes)] Purified Water, Sugar Water, Ethanol
[Non-conductors] Solid Salt, Solid Sugar
From these results, it’s clear that solutions of the electrolytes—salt, hydrogen chloride, and sodium hydroxide—conduct electricity well.
The most fascinating finding here is that solid salt does not conduct electricity, but it becomes conductive when dissolved in water. This is because in the solid state, the ions are rigidly fixed in a crystal lattice and cannot move. When dissolved in water, the ions are released and become free to roam. Even the electric carriers (ions) can’t perform their duty without a “pathway” (= water) that allows them to move freely.Sharing your class’s results on href might lead to even more interesting discoveries. Beyond the variations in individual results, a clear scientific law is bound to emerge.
The salt and sugar in your kitchen are, when viewed differently, a gateway to a grand world of chemistry. There might be scientific secrets hidden all around you that you haven’t noticed yet!
Inquiries and Requests
Make the wonder and fun of science more accessible! We’ve compiled easy-to-understand tips and fun science experiments you can do at home. Try searching around! \href \href \href


