Crystal Clear Science: Making Crystals in Your Kitchen to See How Volcanic and Plutonic Rocks Are Born!
Hello! I’m Ken Kuwako, your Science Trainer. Every Day is an Experiment!
Have you ever taken a really close look at a rock on the side of the road? Some sparkle with large, glittering grains, while others are dull with tiny specks. Believe it or not, these visual differences hide a grand drama deep within the Earth, where hot magma cools and solidifies. In middle school science, we learn about “igneous rocks” – rocks formed when magma cools and hardens. But why do “volcanic rocks” and “plutonic rocks” look so different?
The key to unlocking this mystery lies in the “difference in how magma cools.” This time, I’m thrilled to share a special crystallization experiment that unravels the secrets of rock formation using sodium thiosulfate (commonly known as hypo or dechlorinator), which you can easily find at home!
Through this experiment, you’ll compare how crystals form when cooled quickly versus slowly. The difference in texture between volcanic rocks (porphyritic texture) and plutonic rocks (granular texture) will magically appear right before your eyes!
One type of volcanic rock (andesite).
It has scattered large crystals within an aggregate of smaller crystals.

One type of plutonic rock (diorite).
Crystals of roughly the same size are densely interlocked.

I faced a lot of trial and error at first, but after many experiments, I got the hang of it, and now it’s a guaranteed success in my classes. In this article, I’ll share the preparations, procedures, and the “magic touch” for success, all based on my experience!
What You’ll Need for the Experiment (You can find these at home or in the science lab!)
※All items are relatively easy to find in a science classroom or at home.
- Sodium Thiosulfate (Hypo): Available at 100-yen shops, home centers, or online as a dechlorinator for goldfish.
- Petri Dishes: 2 of them
- Electric Pot or Hot Plate: For melting the hypo in a hot water bath.
- Chopsticks: For stirring.
- Work Gloves: Absolutely essential to prevent burns.
- Container with Ice Water: For rapid cooling.
- Styrofoam Box: For insulation to allow slow cooling.

You can find it on Rakuten here: GEX Dechlorinator Hypo 30g x 4 bags, Dechlorinator, Kanto Same-Day Shipping
Let the Experiment Begin! Recreating Earth’s Internal Drama
Objective:
Visually confirm that the cooling rate determines crystal size!
- Rapid Cooling with Ice Water → Observe the formation of many small crystals (Volcanic Rock Model)
- Slow Cooling with Styrofoam → Observe the growth of large crystals (Plutonic Rock Model)
Experiment Steps:
Melt the Hypo
Fill the petri dishes with hypo until the bottom is covered. Place them in an electric pot with a little water for a “hot water bath” to warm them. Sodium thiosulfate has a low melting point of about 48°C, so it will quickly turn into a clear liquid. Stir gently with chopsticks to dissolve it completely.

Divide into Two Teams
Once liquid, immediately remove from heat. Wearing work gloves to prevent burns, place one petri dish on the ice water and the other inside the styrofoam box.


The Key to Success! Sprinkle “Magic Powder”
This is the most crucial point! Sprinkle a small amount of crushed hypo powder into both petri dishes. These will act as “crystal nuclei,” the “seeds” for crystal growth. Without this step, the liquid might enter a mysterious state called “supercooling,” where it loses its trigger to solidify, and no crystals might form even after 20 minutes!


Careful Observation Time
Now, just wait for about 15-20 minutes! Observe how the crystals grow in the petri dishes, almost like living things. What differences can you see in their formation? I even made a time-lapse video of the crystallization process. You can see 20 minutes of change in just 1 minute!
Please watch this video.
Amazing Results! This is Earth’s Miniature Model
Now, let’s look at the results. On the left is the plutonic rock model, cooled slowly, and on the right is the volcanic rock model, cooled rapidly.
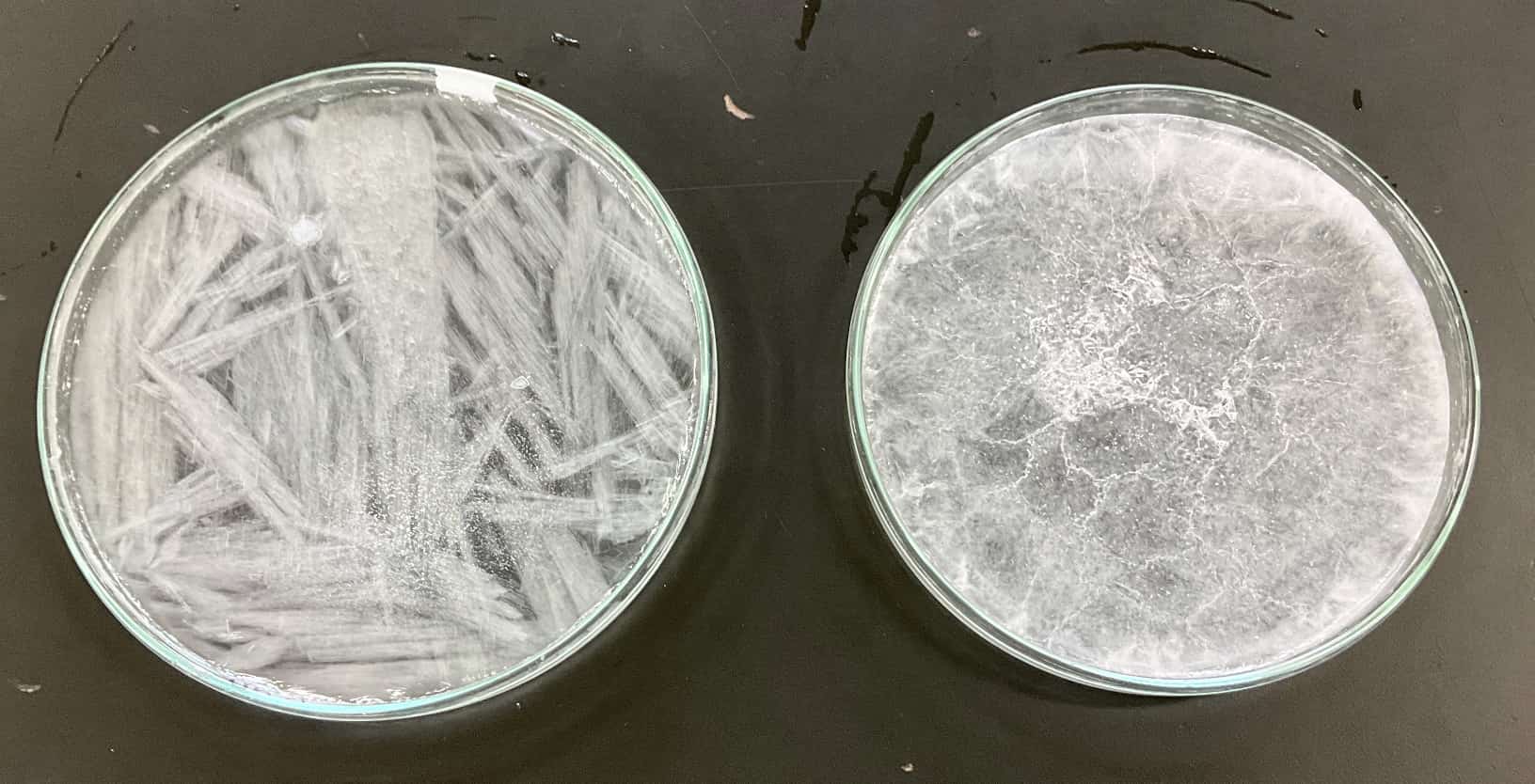
▼Rapidly Cooled (Volcanic Rock Model)
Fine, small crystals have formed all over. When magma cools rapidly near the Earth’s surface, atoms don’t have enough time to align slowly, resulting in only small crystals. This is exactly how “volcanic rocks” form!
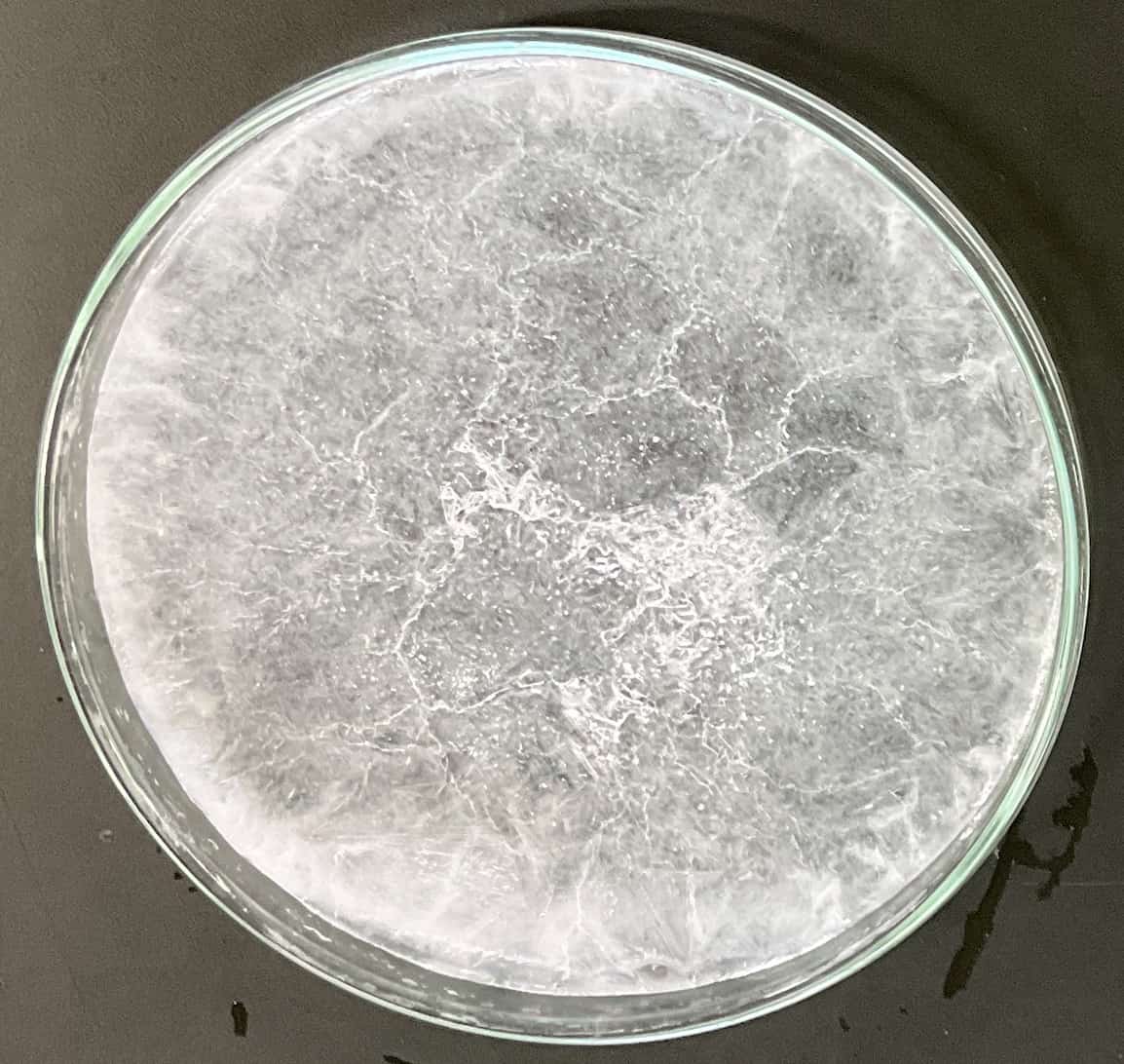
▼Slowly Cooled (Plutonic Rock Model)
Look at this! Long, large, needle-like crystals have grown freely. This is the same principle behind the formation of “plutonic rocks,” where magma cools slowly deep underground over a long period, allowing atoms to grow large.

Tips for Success in the Classroom
- Before the experiment, ask, “What kind of rocks do you think would form if the cooling rate was different?” This helps students observe with a sense of purpose.
- This experiment, from preparation to clean-up, can be easily completed within a typical class period (40-50 minutes).
How was it? With just a little preparation and a few procedural tips, you can dramatically recreate the Earth-scale phenomenon of igneous rock formation right in a petri dish! This experiment, which sparks intellectual excitement and understanding in students, will surely shatter the idea that “science experiments are difficult and require special equipment.” Please consider incorporating it into your fun science lessons!
Inquiries and Requests
Discover the wonders and fun of science up close! I’ve put together easy-to-understand guides on enjoyable science experiments you can do at home, along with tips for success. Please search around!
・About the operator, Ken Kuwako: Click here
・For various requests (writing, lectures, experiment classes, TV supervision, appearances, etc.): Click here
・Article updates are distributed on X!
![]() Science Neta Channel streams experiment videos!
Science Neta Channel streams experiment videos!
2月のイチオシ実験!梱包材で遊ぼう!
- 静電気の時期になってきました。子供と一緒に梱包材で盛り上がろう!→ やめられなくなる!静電気実験20
 体中に梱包材をはりつけてみよう!
体中に梱包材をはりつけてみよう!
テレビ番組等・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師等・ショー・その他お知らせ
- 2/20(金)「生徒の進学希望実現支援事業」研究授業@福井県立若狭高等学校 講師
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) サイエンスショー予定
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。


