The Big Bang: When Hydrogen and Oxygen Ignite! A Dazzling Chemical Reaction That’ll Blow Your Mind.
I’m Ken Kuwako, a science trainer. Every day is an experiment.
【This Article is also a Podcast!】
Experience the Power of Detonating Gas! A Chemical Reaction of Sound and Light
In the science lab, when students get to work with “hydrogen” and “oxygen,” their eyes light up. An invisible gas that makes a “pop!” sound and ignites when a flame is brought near is amazing enough. But when you ask them, “What happens if we mix hydrogen and oxygen?”, you’re met with looks of pure excitement. With proper preparation and safety precautions, this experiment becomes an unforgettable learning experience.
① Gas Properties: What Happens When You Ignite Hydrogen or Oxygen?
First, let’s review the basics.
• When you collect only hydrogen and bring a flame close: It burns with a small “pop” (combining with oxygen to form water).
• When you collect only oxygen and bring a lit incense stick close: The flame gets bigger, demonstrating oxygen’s ability to support combustion.
These are fundamental, safe experiments that get students fully engaged.
② Detonating Gas – The Real Deal!
Now for the main event: detonating gas. This is a mixture of hydrogen and oxygen in a perfect 2:1 volume ratio. When you ignite this gas, BOOM!
First, we need a specially designed rubber stopper to seal the gas.

The rubber stopper has a three-way stopcock, allowing you to easily add and remove gases. Using the stopcock and a syringe, we remove all the air from the bag. Then, we add 6cm³ of oxygen and 12cm³ of hydrogen into the bag and close the stopcock.

|
|
|
|
Two insect pins are inserted into the rubber stopper, and a device for discharging electricity, like a lighter, is connected to the pins. When you press the lighter’s switch,
BOOM!
the hydrogen and oxygen combine. The highlights are the mist that forms inside the bag after the experiment and how the bag collapses. (In the video, the rubber stopper flies off, but that’s part of the fun!)
③ My Epic Fail: The Detonating Soap Bubble Experiment
In the past, I tried an experiment where we put detonating gas inside soap bubbles and ignited them. It was visually spectacular and loud, and the students loved it. However, my carelessness led to a major mistake…

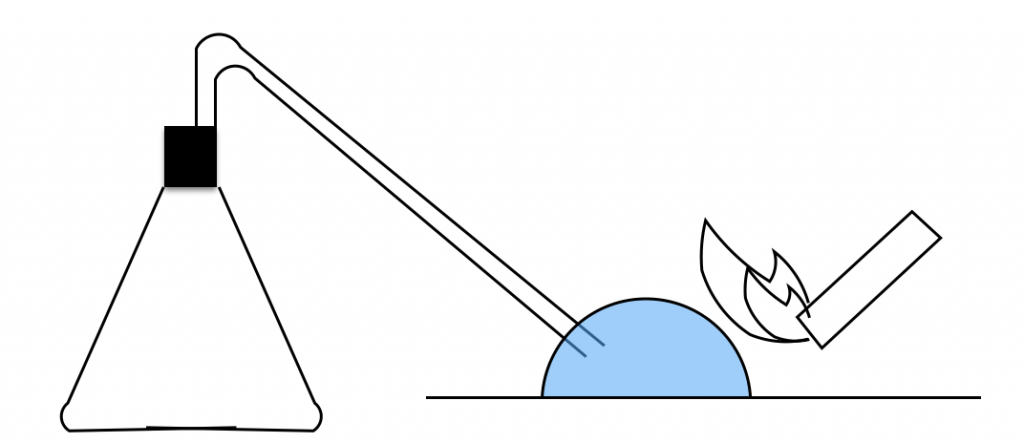
One time, I brought the flame close without removing the rubber tube first. The whole apparatus exploded!
The flask jumped and the equipment was blown apart. Luckily, no one was hurt, but I’ll never forget the cold sweat I broke into. The golden rule for detonating gas is “never ignite it while it’s still in the apparatus.”
④ A Safer Method: Igniting Detonating Gas in a Hose
I highly recommend this method: fill a clear hose with detonating gas and ignite it from one end. It’s safe, impressive, and perfect for science events or classroom demonstrations.
Here’s what you need: a hose (12mm inner diameter, 8m long, you can buy it by the meter at stores like Joyful Honda), a rubber stopper (No. 1 fits perfectly), hydrogen gas cylinder, oxygen gas cylinder, an umbrella bag, a lighter, earplugs, and safety glasses.
Be aware that the hydrogen cylinder runs out quickly. For this experiment, I found that one cylinder is good for about three uses.

I used a hose with a 12mm inner diameter. This one is 10m long, which is a bit long, so I cut it down to 8m. That’s a 2m cut.

Amazon. On Rakuten, you can find it here: TRUSCO Clear Hose 12×15 10m cut (1 roll) TTM-1215C10
It’s also available at home centers. They sold it by the meter. I could have gone for 10m, but 8m felt right. The rubber can degrade, so it’s best to use a new one.


A No. 1 rubber stopper fits this perfectly.


First, we divide the umbrella bag into three equal sections and mark the 2:1 ratio. We fill it with oxygen to the “1” mark and then add hydrogen to the “2” mark. This mixture is our detonating gas. Next, attach the tip of the umbrella bag to the hose and slowly fold the bag to transfer the mixed gas into the hose. Do not cap the hose with the stopper yet. Once all the gas is in the hose,
Cap one end with the rubber stopper.
Insert the lighter into the other end.
Now you’re ready! On the count of “3, 2, 1,” pull the lighter’s trigger to ignite a powerful chemical reaction with a loud sound and a flash of light inside.
Be careful as it makes a loud bang! Cover your ears as a precaution and always wear safety glasses.



You can observe the light and sound, the phenomenon of an explosion, and the stopper on the opposite end shooting off with force as the gas expands from the heat. You can also see condensation form inside the hose, indicating that water was produced. For a more definitive result, you could test it with cobalt chloride paper.
Inquiries and Requests
We want to make the wonders of science more accessible! We’ve put together fun and easy-to-understand science experiments you can do at home, along with tips and tricks. Feel free to search our blog!
• About the operator, Ken Kuwako: Click here
• For requests (writing, lectures, science classes, TV supervision, appearances, etc.): Click here
• For article updates, follow us on X!
![]() Our Science Trick Channel features experiment videos!
Our Science Trick Channel features experiment videos!

![[Product prices may have changed since the link was created.] [Product prices may have changed since the link was created.]](https://hbb.afl.rakuten.co.jp/hgb/29c2e810.62af8e5c.29c2e811.cc00c346/?me_id=1303884&item_id=10001426&pc=https%3A%2F%2Fthumbnail.image.rakuten.co.jp%2F%400_mall%2Fsuzumori%2Fcabinet%2Fexperiments%2Fscience%2Ff35-1905_1.jpg%3F_ex%3D240x240&s=240x240&t=picttext)

![[Product prices may have changed since the link was created.] [Product prices may have changed since the link was created.]](https://hbb.afl.rakuten.co.jp/hgb/29c2e810.62af8e5c.29c2e811.cc00c346/?me_id=1303884&item_id=10005628&pc=https%3A%2F%2Fthumbnail.image.rakuten.co.jp%2F%400_mall%2Fsuzumori%2Fcabinet%2F1610%2Ff35-1921.jpg%3F_ex%3D240x240&s=240x240&t=picttext)

