Ignite with Air Power! Feel the Explosive Force of Adiabatic Compression with the “Fire Piston” Experiment
I’m Ken Kuwako, your Science Trainer. Every day is an experiment.
What if I told you there’s a way to instantly create fire using only the power of air, without a single match or lighter? It sounds like magic, but this is a genuine science experiment. Today, I’m introducing you to an amazing device that utilizes the properties of air: the Fire Syringe (or “Compressed Igniter”).
Seeing is believing. First, take a look at the experiment in action in the video below.
Pretty cool, right? That “Poff!” sound, and a flame instantly burst forth. What secret is hidden behind this incredible phenomenon? Let’s follow the steps of the experiment and unlock the mystery.
The Quick Draw! How the Experiment Works
The mechanism of this device is incredibly simple. It’s just a transparent cylinder and a piston that you plunge inside. First, you tear off a small piece of highly flammable paper (like tissue paper) and place it at the bottom of the sealed glass tube.

From this point on, things get a bit dangerous, so proceed with caution. We attach a transparent protective cover around the cylinder to prevent glass from shattering and scattering due to the rapid pressure change.

Next, we securely set the “lid” (the piston) into place from the top, and we’re ready to go.

Now for the main event. The key is to plunge the piston in: “Without hesitation, all at once, and with force.” If you’re timid and push slowly, the paper won’t ignite. Prepare yourself mentally… and GO!!
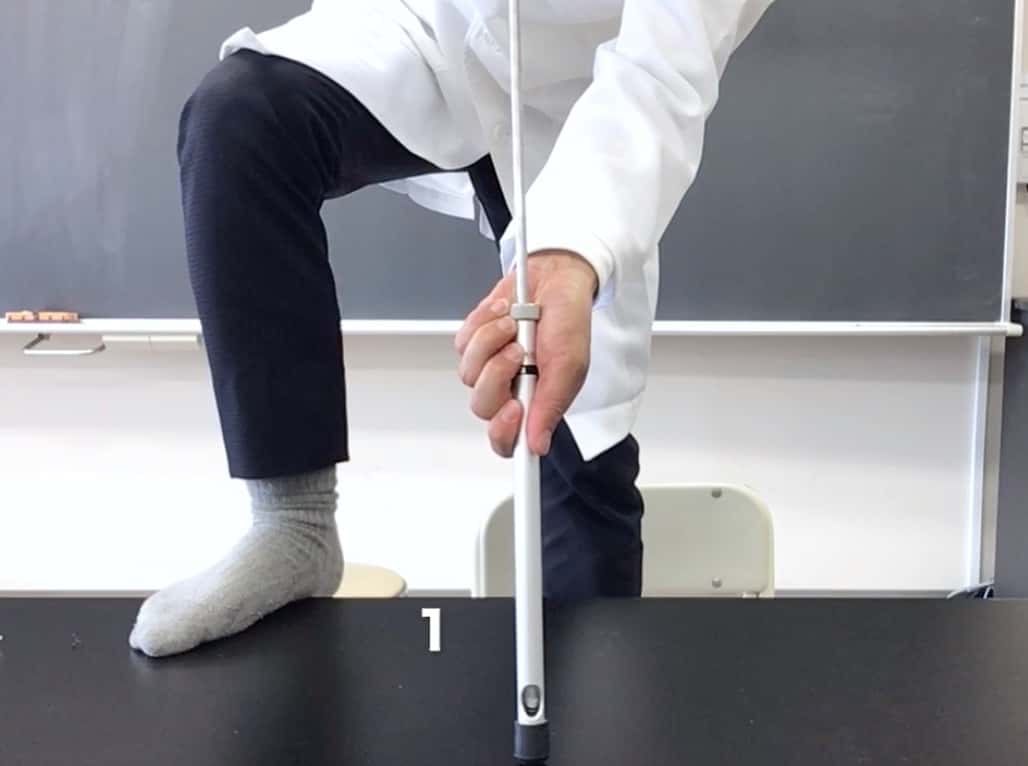
And then, amazingly!

IT BURNED!!
A red flash of light runs through the cylinder for an instant, and smoke fills the tube. No matter how many times I do this, the moment still surprises me. Even as a science teacher who knows the result, there’s something genuinely moving about witnessing fire being created right before your eyes. You can actually make this device with common, household materials! Here is a video of a homemade Fire Syringe.
Why Did It Ignite? Unveiling the Science
The paper ignited without using any heating tools. How is that possible? The key is Air Compression. In this experiment, we compressed the gas so rapidly that heat didn’t have time to enter or leave the system. In physics, this process is called Adiabatic Compression.
Picture this: If you suddenly cram a lot of people into an already packed train car, the inside quickly becomes chaotic, and the temperature rises, right? It’s the same principle with air. When air is forced into a much smaller space, the air molecules collide violently, and that energy is converted directly into “heat”. At that moment, the temperature inside the cylinder soars past the ignition point of the paper.
Decoded by High School Physics (The First Law of Thermodynamics)
We can understand this phenomenon much more clearly by expressing it using an equation taught in high school physics: The First Law of Thermodynamics.
Q = ΔU + W
Heat Transferred = Change in Internal Energy + Work Done by the Gas
In this case, because the compression happens instantly, there is no heat transfer (it’s adiabatic). Therefore, Q = 0. Let’s rearrange the equation.
0 = ΔU + W
ーW = ΔU
Here, “−W” means that “Work was done on the gas” (i.e., the human pushed the piston). This means that “The energy (work) the human put into the system by pushing hard was directly converted into an increase in the gas’s internal energy (temperature).”
Contact and Requests
Let’s bring the wonder and fun of science closer to you! We’ve compiled easy-to-understand tips and exciting science experiments you can do at home. Feel free to search around! ・The content of “Science Note” has been published as a book. Details here ・About the operator, Ken Kuwako: Find out more here. ・For various requests (writing, lectures, experimental classes, TV supervision/appearances, etc.): Contact us here. ・Article updates are posted on X!
![]() We distribute experimental videos on the Science Note Channel!
We distribute experimental videos on the Science Note Channel!
3月のイチオシ実験!
- 押し花を作ろう!:梅や桜の花の押し花を作ってみましょう。特別なケースに入れると、長く保存できて、しおりにもなります。
テレビ番組・科学監修等のお知らせ
- 「月曜から夜更かし」(日本テレビ)にて科学監修・出演しました。
- 2月27日放送予定「チコちゃんに叱られる」(NHK)の科学監修しました。
書籍のお知らせ
- 1/27 『見えない力と遊ぼう!電気・磁石・熱の実験』(工学社)を執筆しました。
- サクセス15 2月号にて「浸透圧」に関する科学記事を執筆しました。
- 『大人のための高校物理復習帳』(講談社)…一般向けに日常の物理について公式を元に紐解きました。特設サイトでは実験を多数紹介しています。※増刷がかかり6刷となりました(2026/02/01)

- 『きめる!共通テスト 物理基礎 改訂版』(学研)… 高校物理の参考書です。イラストを多くしてイメージが持てるように描きました。授業についていけない、物理が苦手、そんな生徒におすすめです。特設サイトはこちら。

講師・ショー・その他お知らせ
- 3/20(金) 日本理科教育学会オンライン全国大会2026「慣性の法則の概念形成を目指した探究的な学びの実践」について発表します。B会場 第3セッション: 学習指導・教材(中学校)③ 11:20-12:20
- 7/18(土) 教員向け実験講習会「ナリカカサイエンスアカデミー」の講師をします。お会いしましょう。
- 10/10(土) 秘密兵器「帯電ガン」が炸裂!ビリビリ!ドキドキ!静電気サイエンスショー@千葉市科学フェスタ(午後予定)
- 各種SNS X(Twitter)/instagram/Facebook/BlueSky/Threads
Explore
- 楽しい実験…お子さんと一緒に夢中になれるイチオシの科学実験を多数紹介しています。また、高校物理の理解を深めるための動画教材も用意しました。
- 理科の教材… 理科教師をバックアップ!授業の質を高め、準備を効率化するための選りすぐりの教材を紹介しています。
- Youtube…科学実験等の動画を配信しています。
- 科学ラジオ …科学トピックをほぼ毎日配信中!AI技術を駆使して作成した「耳で楽しむ科学」をお届けします。
- 講演 …全国各地で実験講習会・サイエンスショー等を行っています。
- About …「科学のネタ帳」のコンセプトや、運営者である桑子研のプロフィール・想いをまとめています。
- お問い合わせ …実験教室のご依頼、執筆・講演の相談、科学監修等はこちらのフォームからお寄せください。



