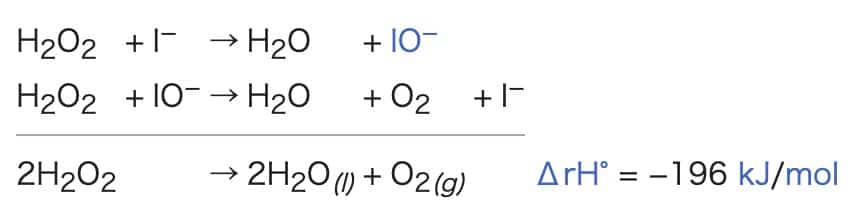Hugely Popular at Science Shows! Find the Optimal Formula for Elephant’s Toothpaste 🐘!
Have you ever heard of an experiment called “Elephant’s Toothpaste“? When the experiment begins, a massive amount of foam erupts, looking just like giant toothpaste. It’s a visually impactful experiment that’s popular in science classes and science shows.
This time, we did the “Elephant’s Toothpaste” experiment with students. To see the reaction for yourself, please watch the video below!
To make the science show a success, we considered the best and safest proportions for the Elephant’s Toothpaste to expand well. Hydrogen peroxide constantly decomposes into oxygen and water, which is why it’s stored in a refrigerator. However, if left alone, its decomposition rate is extremely slow.
In this experiment, by adding potassium iodide (KI) to hydrogen peroxide (H₂O₂), a large amount of oxygen is rapidly generated, creating a large volume of foam. Potassium iodide acts as a catalyst, instantly accelerating the decomposition of hydrogen peroxide. The chemical reaction is as follows:
The reaction equation appears to be as follows:
This is it:
2H2O2 → 2H2O + O2
The size and vigor of the foam depend on the concentration and amount of hydrogen peroxide used. The higher the concentration of hydrogen peroxide, the faster the reaction and the more powerful the foam. However, if the reaction is too violent, it becomes difficult to control, which is the challenging part of this experiment. We conduct these experiments with safety in mind.
This chemical reaction is also an exothermic reaction, and you will notice that the foam and the container feel warm to the touch after the experiment. Beyond the visual impact, you can learn about chemical concepts like reaction heat and catalysts.
Specific Experimental Methods
We tried three methods.
① Use 50 mL of hydrogen peroxide and dissolve 3 tablespoonfuls of potassium iodide in 50 mL of hot water.
Pour 50 mL of hydrogen peroxide into a 500 mL Erlenmeyer flask, and add dish soap and, if you want to color it, food coloring. Place this in a basin, then add the potassium iodide dissolved in water (3 tablespoonfuls from a chemical spoon).

When you add the potassium iodide, a rapid generation of oxygen occurs, and thanks to the dish soap, you get soap bubbles.


With 50 mL, the foam comes out and fills up the basin. The amount of foam is determined by the amount of hydrogen peroxide.


Reference: Use 50 mL of hydrogen peroxide and add 3 tablespoonfuls of undissolved potassium iodide.
When potassium iodide is added as a solid without dissolving it in water, it came out like a “snake firework.” The reaction is slower and you can observe it for a longer time.

Also, be careful, as the solution can splatter if you do the experiment without adding dish soap. When we took a small amount of hydrogen peroxide, about 5 mL, in a graduated cylinder and added a little potassium iodide (one scoop with the small chemical spoon), and then inserted an incense stick, it flared up with a small flame, showing that oxygen was being generated.

② Use 200 mL of hydrogen peroxide and dissolve 6 tablespoonfuls of potassium iodide in 50 mL of hot water.
We added 200 mL of hydrogen peroxide and potassium iodide dissolved in 50 mL of hot water (6 tablespoonfuls from a chemical spoon). Here’s what it looked like.

200 mL of hydrogen peroxide
It spread all over the table.

I think this is a pretty good amount. But I regret using too much water to dissolve the potassium iodide.
Use 200 mL of hydrogen peroxide and dissolve 6 tablespoonfuls of potassium iodide in 30 mL of hot water.
We added 200 mL of hydrogen peroxide and potassium iodide dissolved in 30 mL of hot water (6 tablespoonfuls from a chemical spoon). Here’s what it looked like.

It still covered the entire table.
The dish soap we used was this one, from Kyu-Kyu-Tto.

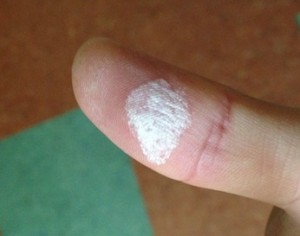
By the way, diluted hydrogen peroxide is called oxygenated water, but while it’s safe when diluted, high-concentration hydrogen peroxide is dangerous if it touches your skin. You might be tempted to touch the foam, but this is also dangerous. Your skin will turn white and sting for a day. Be sure to wear gloves, a lab coat, and safety goggles. It’s best for a teacher to handle the cleanup.
I actually touched it while cleaning up. My skin turned white. Also, absolutely do not touch the foam that is generated afterwards. This foam also causes a stinging sensation when touched. Please be careful.
If you touch it, your skin will change color. This is what happened to me when I actually touched it. A human experiment. The stinging sensation lasted for a long time.
Inquiries and Requests
Discover the wonder and fun of science! We’ve put together easy-to-understand explanations of fun science experiments you can do at home, along with tips. Feel free to search for various things!
・About the operator, Ken Kuwako: click here
・For various requests (writing, lectures, experiment workshops, TV supervision, appearances, etc.): click here
・Updates on articles are available on X!
![]() Kuwako’s Science Channel posts experiment videos!
Kuwako’s Science Channel posts experiment videos!